Pulmonary hypertensive changes are commonly seen by the surgical pathologist, but the majority represents secondary changes due to some process extrinsic to the lung. Some primary, or idiopathic, vascular diseases result in unique pathologic changes including the plexiform lesion and venous hypertensive changes. Thromboembolic disease also shows unique pathologic features. Diffuse alveolar hemorrhage, vasculitis, and capillaritis often overlap, but may represent separate, distinct pathologic processes. Lastly, alveolar capillary dysplasia with misalignment of pulmonary veins, as well as chronic lung allograft vasculopathy, present as unique pathologies in the neonate and posttransplant recipient, respectively.
Key points
- •
Pulmonary hypertensive changes result in vascular (usually arterial) remodeling.
- •
Pulmonary hypertensive changes are mostly nonspecific from a histologic perspective, with a few notable exceptions (plexiform lesions, veno-occlusive disease, and granulomatosis with polyangiitis).
- •
Pulmonary vascular disease can also be observed in the neonate and posttransplant settings.
Introduction
Pulmonary vascular disease is typically encountered by the surgical pathologist as a pathologic finding present in the background of some other disease process. A lobectomy for lung cancer or a bullectomy for emphysematous lungs will often show pathologic changes if one goes and examines the vasculature. Most of these changes represent hypertensive changes secondary to some other process, but pathologists may occasionally encounter specimens biopsied or resected for a primary vascular pathology. For simplicity and for the purposes of this review, pulmonary vascular disease is discussed in 4 broad topics: (1) pulmonary hypertension (PAH)—including those conditions arising primary in the lung and changes associated with secondary causes, (2) vasculitis and diffuse alveolar hemorrhage (DAH), (3) congenital and pediatric lesions, and (4) posttransplant vascular changes. The level of detail required to fully discuss the topics in this review deserve standalone articles or individual book chapters, but this is not possible when preparing a succinct review. The discussion of these pathologic entities has been simplified for the purposes of this review and to accommodate the most likely consumer of this review, a radiologist. The main pathologic findings are drawn from numerous other studies and are summarized in this text. The reader is referred to these references and reviews if additional details not covered in this text are desired. This article will feature histopathologic changes of PAH and, when applicable, will include corresponding imaging findings, but detailed discussion on imaging will be discussed in subsequent articles.
Pulmonary hypertension
Introduction
The pulmonary vascular network is characterized by low vascular resistance, typically with pressures one-eighth that of systemic pressures. PAH (PAH will be used throughout this text as the majority of hypertensive change is observed in the pulmonary arterial system) is a progressive disease clinically defined as the state in which pressure in the pulmonary artery is increased above a mean of 20 to 25 mm Hg at rest, regardless of the underlying mechanism. It is divided into 5 groups based upon World Health Organization (WHO) classification ( Box 1 ). Patients usually present with symptoms consistent with right ventricular failure such a dyspnea, fatigue, syncope, edema, abdominal pain, and chest pain. In its severe form, PAH may lead to right heart failure and death without adequate treatment. Hypertension in the pulmonary circulation can also occur in physiologic settings, but only transiently, such as with exercise. A recent systematic review based on multinational data by Emmons-Bell and colleagues reported an incidence of 0.0008 to 1.4 cases and prevalence of 0.37 to 15/100,000 person-years.
Group 1: Pulmonary arterial hypertension (PAH)
Group 2: Pulmonary hypertension due to left heart disease
Group 3: Pulmonary hypertension due to lung disease and/or hypoxia
Group 4: Chronic thromboembolic pulmonary hypertension (CTEPH)
Group 5: Pulmonary hypertension with unclear or multifactorial mechanisms
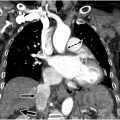
Radiographic findings of PAH are not uncommonly encountered and are briefly reviewed here. , , Classic findings on chest radiographs are usually present in advanced disease: enlarged heart, elevated cardiac apex due to right ventricular hypertrophy, central pulmonary artery dilation, and reduced retrosternal clear space from right ventricular and pulmonary artery enlargement. On chest computed tomography (CT), dilated central and peripheral pulmonary arteries along with end diastolic right ventricular wall thickness less than 4 mm, right ventricular dilation, inferior vena cava and hepatic vein dilation, and centrilobular ground-glass attenuation nodules may be present. Echocardiography is used to assess right ventricular function by measuring systolic pulmonary arterial pressure and tricuspid regurgitation. Cardiac MR imaging is the reference standard for right ventricular function.
The most encountered vascular pathology observed by the practicing surgical pathologist is PAH arising from some secondary process. Most often these are secondary changes observed in the pulmonary arterial system secondary to left heart failure or other conditions. Hypertensive changes are also common in interstitial lung diseases. There are rare cases of primary PAH (now more accurately termed idiopathic PAH), but these are less often seen by the surgical pathologist as primary PAH clinical management has improved in recent years. Pulmonary hypertensive changes may also be observed in the venous system, but conditions that lead to venous hypertension are less commonly observed in surgical pathology. Different histologic classification and grading systems for pulmonary hypertensive change have been developed for PAH ( Table 1 ), but the original grading system is infrequently used in clinical sign-out as many of these histologic changes are rarely encountered, and only grades I, II, and III are seen in standard cases of secondary PAH. , Regardless of the type of PAH and the etiology, destructive remodeling of the vascular bed is observed. These changes are often observed in smaller branches of the pulmonary arteries and consist of either intimal lesions (fibrosis and thickening ( Figs. 1 A, B and 2 ) or plexiform changes), medial (muscular) hypertrophy/hyperplasia (see Fig. 2 A, B), or a combination of these changes with or without adventitial changes. ,
| Intimal Changes | Medial Changes | Degree of Severity | |
|---|---|---|---|
| Grade 1 | — | Hypertrophy | Mild-to-moderate pulmonary hypertension |
| Grade 2 | Cellular proliferation | Hypertrophy | |
| Grade 3 | Fibrosis | Hypertrophy; Some vascular dilation | |
| Grade 4 | Fibrosis | Hypertrophy; Progressive dilation | |
| Grade 5 | Fibrosis and plexiform lesions | Hypertrophy; Vascular dilation; and hemosiderin deposition | Severe pulmonary hypertension |
| Grade 6 | Fibrosis and plexiform lesions | Hypertrophy; Vascular dilation; hemosiderin deposition; and necrotizing arteritis |


Brief Overview of Idiopathic (Primary) Pulmonary Arterial Hypertension
Primary PAH (group 1) includes PAH that arises idiopathically, or from heritable conditions, drugs and/or toxins, or from known associations such as connective tissue diseases, portal hypertension (cirrhosis), or infection (most notably, human immunodeficiency virus). The main histologic finding that separates these forms of PAH from others is the presence of plexiform lesions. While plexiform lesions can be observed across all forms of PAH, their presence strongly suggests a primary (idiopathic) form of PAH. Two other conditions have been classically linked to this group but are distinct from the other conditions in group 1 PAH and show lesions in the venous circulation. These unique conditions include pulmonary veno-occlusive disease (PVOD) and pulmonary capillary hemangiomatosis (PCH). PVOD and PCH both often present with congestive changes and hemosiderin-laden macrophages. Significant fibrous occlusion of veins is diagnostic of PVOD, while capillary proliferations within alveolar septa, near intralobular septa, and near bronchovascular bundles are common in PCH. PVOD and PCH may be seen together, and some view these conditions as a similar disease occurring on a spectrum, which is supported by a more recently described common genetic background of the 2 disease processes.
Brief Overview of Secondary Pulmonary Arterial Hypertension
Nonidiopathic PAH arises from various secondary processes: left heart disease (group 2), lung diseases and/or hypoxia (group 3), and chronic thromboembolic disease (group 4). Similar to primary PAH (group 1), there is often medial hypertrophy and intimal fibrosis. Muscularization of arterioles and plexiform lesions are typically absent in this group, as are congestive changes and increased numbers of hemosiderin-laden alveolar macrophages.
Pathologic Findings in Pulmonary Hypertension
Nearly all forms of PAH show medial and intimal hyperplasia. In mild-to-moderate PAH, there is medial hypertrophy of medium-sized muscular arteries with some degree of intimal thickening or fibrosis. In severe forms of PAH, there is often significant luminal narrowing from concentric intimal fibrosis ( Fig. 3 ); plexiform and angiomatoid lesions, and necrotizing arteritis, may also be seen in the most severe forms but are not common findings in routine surgical pathology specimens.

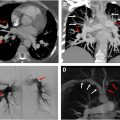
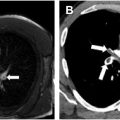
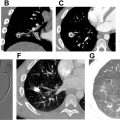
While the changes of medial and intimal hyperplasia are present in all forms of PAH, there are subtle clues that can indicate the cause of the PAH. Plexiform (and angiomatoid) lesions ( Figs. 4 A, B and 5 A, B ) should prompt the pathologist to consider idiopathic PAH. Plexiform lesions are found in muscular arteries. Plexiform lesions are thought to arise from vascular occlusion, breakdown of elastic layers resulting in endothelial cell proliferation and neolumen formation along with collateralization of vessels. , Fibrous occlusion of veins ( Figs. 6 and 7 A, B ) is nearly pathognomonic for PVOD. Pulmonary veins are located in the interlobular septa, and occlusion of veins can be quite subtle. Given that there is venous occlusion, this often results in congestion in the arterial system. Congestion is also typical of PCH ( Figs. 8 A, B and 9 A, B ). Due to the chronic congestion, it is typical to see numerous intra-alveolar hemosiderin-laden macrophages ( Fig. 10 ) or alveolar hemorrhage. Expert thoracic pathologists often caution rendering a diagnosis of PVOD or PCH in the absence of significant hemosiderin-laden macrophages or hemorrhage. Another secondary cause of PAH that is easily identifiable under the microscope are changes associated with thromboembolic disease. Thromboembolic disease often results in arterial system thrombi (recent fibrin thrombi with or without organization [ Figs. 11 A, B and 12 ]), and recanalization (see Fig. 12 A, B) can be seen in older lesions. In acute pulmonary embolism ( Fig. 13 ), changes of infarction may also be identified, but this may be a challenging diagnosis in biopsies, due to the multiple pathologic changes that may be observed in infarctions including a pseudogranulomatous response. Eccentric intimal fibrosis is a subtle clue that thromboembolic disease may be the cause of PAH. Intravascular foreign material (often easily identified under polarized light), is a form of embolic disease, often seen in patients with a history of intravenous (IV) drug use. A similar phenomena to IV drug use is the recent identification of hydrophilic polymer in the vascular system following numerous intravascular cannulations ( Fig. 14 ). ,



Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree