Traditionally, the abdominal cavity has been divided into a number of peritoneal, retroperitoneal, and extraperitoneal spaces. Although it is useful for learning abdominal anatomy and appreciating the confinement of disease in a particular space, this classic approach affords limited understanding of the intra-abdominal spread of disease. Dissemination of disease between the retroperitoneum and the peritoneal cavity, between the subdivisions of the retroperitoneum, and within the subperitoneal spaces is difficult to conceptualize if the various spaces and compartments of the abdomen and pelvis are considered fixed, immutable, and isolated delineators of anatomy. Meyers and colleagues perceived that the abdominal cavity should be viewed rather as a continuous space that is punctuated by the abdominal mesenteries, ligaments, and fascia, which may confine disease or provide an avenue for its spread. Blood vessels, lymphatics, and the biliary system also contribute to the spread of a number of benign and malignant processes. This holistic schema provides a rationale for understanding the dissemination of intra-abdominal disease focally and to areas distant from the site of origin.
Hematogenous Spread
Malignant Disease
Malignant neoplasms shed as many as 4 million cells per gram of tumor tissue into the bloodstream every day. Of these cells, more than 90% are cleared rapidly from the circulation. Despite this metastatic inefficiency, the abdominal organs and gut ( Fig. 109-1 ) are common sites of hematogenous metastases from intra-abdominal and extra-abdominal primary sites.

Liver Metastases
Because of its dual blood supply, the liver is a common site of metastatic disease. The portal system conveys cancer cells from tumors of the colon, pancreas, stomach, and small bowel, whereas the systemic arterial system carries metastases from any site but most often from the lung and breast. The liver is particularly vulnerable to the deposition of metastases because of its architecture. Kupffer cells extend into the hepatic sinusoids and impede the passage of tumor cells. These cells then subendothelially extravasate, indent the hepatocytes, and, because of their position adjacent to the sinusoids, receive a rich mixture of arterial and portal blood. In this favorable milieu, it is not surprising that metastases in the liver grow four to six times faster than those at other sites, that the liver is the second most common site of metastases (after lymph nodes), and that 25% of all patients who die of cancer have liver metastases.
The radiographic appearance of liver metastases depends on the tumor type, its vascularity, and the degree to which neovascularization occurs. Well-established metastases derive most of their blood supply from the hepatic artery, and this fact is important for planning local hepatic chemotherapy or embolization therapy.
Gut Metastases
The hollow viscera are important but less frequent sites of hematogenous metastases. Carcinoma of the breast or lung and melanoma are the most common primary tumors. On occasion, symptoms of bowel metastases, such as obstruction or hemorrhage, may be the initial clinical manifestations of an occult primary neoplasm.
The radiographic appearance of gut metastases depends on the primary tumor type, the degree of vascularity and rate of growth, and the tumor’s ability to induce desmoplasia. Hematogenous metastases have a distinct predilection for the antimesenteric border of the bowel because tumor cells are trapped in the smallest capillaries, which are located on the antimesenteric side of the gut. The mesenteric vessels are larger at their entrance on the mesenteric border. A mass, ulcer, ulcerated mass, or area of stenosis may be seen radiographically.
Infection
Portal Vein
In the preantibiotic era, pyogenic liver abscesses were usually caused by portal vein pyemia resulting from appendicitis, diverticulitis, or pelvic inflammatory disease. In Western countries, portal vein pylephlebitis is now rare, and ascending biliary infections are the most common cause of pyogenic hepatic abscesses.
Approximately 10% of the world’s population are infected by Entamoeba histolytica , and 10% of these infections cause clinical disease. The portal vein remains the principal conduit for invasive amebiasis of the colon. The protozoa gain access to the portal venous system by invading the colonic mucosa, and they are then carried to the liver, where they obstruct small portal and arterial radicles, producing parenchymal infarction and proteolysis with subsequent abscess formation.
The portal vein also transmits the eggs of Echinococcus granulosus to the liver. The eggs are dissolved by the digestive juices, and the released oncospheres pass through the duodenal mucosa into the portal circulation. In the liver, the eggs disintegrate or grow into a hydatid cyst (see Chapter 88 ).
Schistosoma mansoni organisms are also carried to the liver through the portal vein. When the worm burden is sufficient, portal vein obstruction may occur, leading to portal hypertension. Schistosomiasis is the most common cause of portal hypertension in the world.
Systemic Arteries
Before the 1980s, hematogenous dissemination of bacterial infections to the abdomen was becoming less frequent and less lethal. This pathway, however, is making a grim comeback in injection drug users, patients with acquired immunodeficiency syndrome (AIDS), and other immunocompromised persons. Pyogenic and nonpyogenic abscesses (as found in candidiasis, tuberculosis, and Pneumocystis jiroveci and Cryptococcus neoformans infections) occur with greater frequency in these patients.
Lymphatic Spread
Neoplasms of the bile ducts, pancreas, stomach, small bowel, and colon ( Fig. 109-2 ) commonly invade adjacent lymphatics. Although a specific discussion of each malignant neoplasm is beyond the scope of this chapter, several fundamental principles apply. Lymphatic tumor emboli from primary bowel neoplasms are not always arrested in the nearest draining lymph node. Lymphatic obstruction of a more remote node may occur because of cellular impaction. This can cause retrograde tumor spread to an adjacent segment of gut or a more distant portion of the alimentary tract. This may explain the phenomenon of anastomotic recurrence of colon cancer, even though a no-touch technique and wide surgical margins are used during resection.

Tumor-induced derangements of lymph flow produce a number of radiographic findings. In the colon, there may be edema of the bowel wall with mucosal thickening, loss of colonic haustra, and narrowing of the lumen. On computed tomography (CT) scans, increased attenuation of the pericolic fat may be seen. With disease progression, nodular tumor deposits accompany the edema, leading to thumbprinting in the colon and cobblestoning of the small bowel. These radiographic findings, which may simulate ischemic colitis and Crohn’s disease, respectively, indicate extensive lymphatic permeation and suggest that resection will probably not be curative. Lymphatic obstruction by tumor at the root of the small bowel mesentery may cause focal or diffuse thickening of the valvulae conniventes from lymphedema or tumor infiltration. When the lymphatics of the liver are obstructed, periportal lymphedema develops, which may cause zones of periportal lucency on CT scans and periportal hyperintensity on T2-weighted magnetic resonance (MR) images of the liver.
Infection and inflammatory disease can also be spread through lymphatics. Patients with viral hepatitis often show spread of the hepatic inflammatory process to regional lymph nodes (see Chapter 89 ).
Biliary Spread
Infection
The biliary system is a major conduit for infectious disease. Asian cholangiohepatitis is the most common biliary tract disease in parts of China, Japan, and other countries of the Far East. Most affected persons harbor parasites of Clonorchis sinensis or Fasciola hepatica , which may obstruct the bile ducts or act as a nidus for stone formation and chronic inflammation. These liver flukes migrate from the duodenum, through the ampulla of Vater, and on to any part of the biliary tract.
Ascaris lumbricoides affects one fourth of the world’s population. Adult worms often migrate from the duodenum through the ampulla of Vater to lodge in the gallbladder, the biliary tract, or less commonly the pancreatic duct. Patients may present with acute cholecystitis or biliary obstruction. Established liver infections, such as hydatid cysts, may erode through and rupture into the biliary system, and the expelled contents may cause obstructive jaundice (see Chapter 88 ).
Ascending cholangitis is a pyogenic infection of the biliary tract and the most common cause of liver abscess in the Western world. It occurs in the clinical setting of obstruction and biliary stasis proximal to an obstructing stone, stricture, or neoplasm.
Patients with AIDS are subject to a number of biliary and pancreatic duct abnormalities because of direct involvement by organisms such as Cryptococcus and Cryptosporidium , which also migrate from the duodenum through the ampulla. On radiographic evaluation, infection may produce an appearance similar to that of sclerosing cholangitis, papillary stenosis, or acute cholecystitis (see Chapter 80 ).
Malignant Disease
Hepatocellular carcinoma and cholangiocarcinoma uncommonly seed the biliary tract.
Peritoneal Spread
Intraperitoneal Flow of Fluid
The peritoneal cavity contains a number of compartmentalized but communicating spaces (see Chapter 108 ). The peritoneal cavity can be divided into supramesocolic and inframesocolic spaces by the transverse mesocolon. Four intraperitoneal spaces are present in the supramesocolic portion of the abdomen: the suprahepatic and subhepatic spaces on the right and the subphrenic space and lesser sac on the left. The inframesocolic portion of the peritoneal cavity is divided into the right and left inframesocolic spaces by the small bowel mesentery. The large left inframesocolic space is open to the pelvis, except where it is bounded by the sigmoid mesocolon. The right inframesocolic space is smaller and caudally restricted by the junction of the distal small bowel mesentery with the cecum. The most dependent portions of the peritoneal cavity are the lateral paravesical spaces and the pouch of Douglas (i.e., cul-de-sac in women and rectovesical space in men). The pelvis communicates with the supramesocolic space through the paracolic gutters, which lie lateral to the attachments of the peritoneal reflections of the ascending and descending colon. The right paracolic gutter is continuous with the right subhepatic and suprahepatic space and diaphragm. On the left, the phrenicocolic ligament forms a partial barrier between the left paracolic gutter and the left subphrenic space.
The natural flow of intraperitoneal fluid occurs along pathways determined by the anatomic compartmentalization of the peritoneal cavity, intraperitoneal pressure, position of the patient, site of fluid origin, nature of fluid, speed of fluid accumulation, presence of adhesions or previous surgery, degree of bladder distention, and density of fluid ( Fig. 109-3 ). Abscesses form and malignant cells grow where natural flow allows the affected ascitic fluid to pool. Fluid in the inframesocolic space seeks the pelvis but may first seed the superior aspect of the sigmoid colon on the left and the medial aspect of the cecum on the right. In the pelvis, infected and malignant fluid fills the pouch of Douglas and then the lateral paravesical recesses. Pelvic fluid then ascends both paracolic gutters, driven by negative intra-abdominal pressure associated with respiration, volume considerations, and topographic anatomy of peritoneal recesses. The flow in the left paracolic gutter is modest and limited by the phrenicocolic ligament. Most flow occurs in the right paracolic space. Fluid in this gutter has access to Morison’s pouch (the hepatorenal recess of the subhepatic space), the right subphrenic space, and potentially the lesser sac through the anterior subhepatic space and foramen of Winslow. The falciform ligament usually prevents fluid spread across the midline from the right to the left subphrenic spaces.
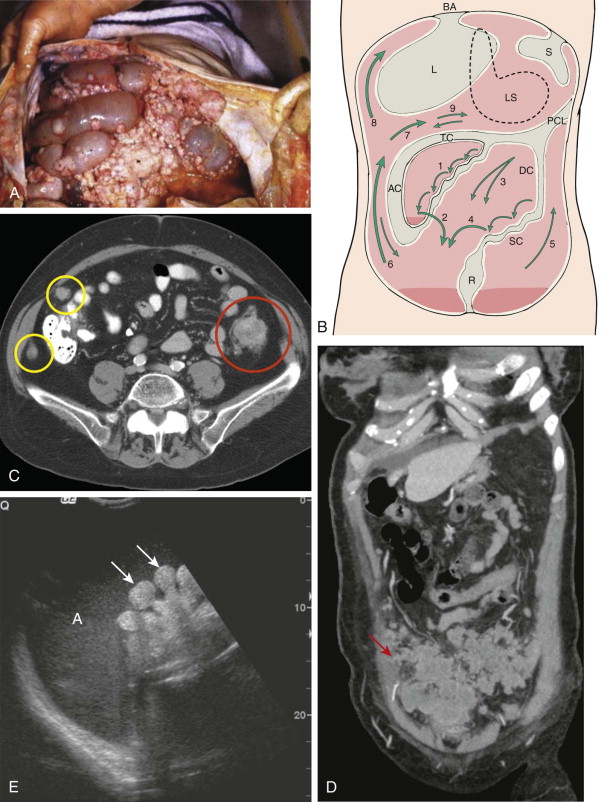
The most common sites of intraperitoneal abscess are the pelvis, the right subhepatic space, and the right subphrenic space. Similarly, the pouch of Douglas, the lower small bowel mesentery near the ileocecal junction, the sigmoid mesocolon, the right paracolic gutter, and the right subhepatic and subphrenic spaces are the most common sites for the growth of seeded peritoneal metastases.
Pouch of Douglas
Malignant Disease
The pouch of Douglas projects at the level of the lower second to fourth sacral segment in most people ( Fig. 109-4 ). On barium studies, drop metastases to this pouch produce a nodular, serosal indentation or fixed parallel folds along the anterior aspect of the rectosigmoid junction. These changes, which clinically correlate with the classic Blumer shelf, reflect the coalescence of tumor deposits with a dense fibrous reaction. This radiographic appearance can be simulated by endometriosis, inflammation of the seminal vesicles, prostate neoplasm or infection, scirrhous colon cancer, or radiation-induced changes.


Infection
Infected fluid naturally pools in the pouch of Douglas, often leading to abscess formation ( Fig. 109-5 ). On plain radiographs, this fluid may be seen as a soft tissue density superior to the urinary bladder. Abscesses may also cause extrinsic distortion of the bladder dome, compress the rectosigmoid junction, or posteriorly and superiorly displace the sigmoid colon.

Perihepatic Spaces
Malignant Disease
Perihepatic dissemination of ovarian carcinoma is commonly seen on CT scans and is manifested as nodular, plaquelike, or sheetlike masses that may be calcified ( Fig. 109-6 ). Free-floating ovarian carcinoma cells are removed from the peritoneum by lymphatic channels in the diaphragm, particularly on the right. The submesothelial lymphatic capillaries of the diaphragm penetrate the muscle to communicate with a similar plexus arising on the pleural surface. From these diaphragmatic lymphatics, lymph drains to the cardiophrenic and mediastinal lymph nodes. Accordingly, a pelvic malignant neoplasm such as ovarian carcinoma can cause adenopathy at the cardiophrenic angle. Malignant ascites and peritoneal implants occur when these lymphatics are obstructed by tumor.



Infection
Right subphrenic and subhepatic abscesses occur two to three times more frequently than left subphrenic and perisplenic abscesses because of the fact that more right-sided surgical procedures, such as cholecystectomy and appendectomy, are performed and because Morison’s pouch acts as a sewer for the right paracolic gutter. Infected fluid on the right side does not extend to the left subphrenic space because it is blocked by the falciform ligament.
Left Subphrenic Space
Left subphrenic space ( Fig. 109-7 ) abscesses most commonly result from perforated anterior gastric or duodenal bulb ulcers or are a sequela of gastric, colonic, or splenic surgery. The negative intra-abdominal pressure beneath the diaphragm preferentially draws infected material toward the diaphragm. The phrenicocolic ligament and falciform ligament prevent transcoelomic spread of generalized peritonitis and tumor.


Lower Small Bowel Mesentery
A series of peritoneal recesses is formed along the right side of the small bowel mesentery as the mesenteric ruffles extend from the root of the mesentery to support the small bowel loops. Disease-bearing fluid and cells cascade from recess to recess toward the right lower quadrant and pool at the level of the distal ileum and cecum before overflowing into the pelvis.
Malignant seeding down the small bowel mesentery ( Fig. 109-8 ) may be manifested as a mesenteric mass, stricture, spiculation, ulceration, or tethering of small bowel loops. If multiple adjacent recesses are affected, palisading of small bowel loops, in which narrowed loops are aligned in parallel configuration, may be seen on barium studies.

Sigmoid Mesocolon
Infected and malignant fluid from the left inframesocolic space collects on the superior aspect of the sigmoid mesocolon ( Fig. 109-9 ). This may cause a serosal mass effect on the superior aspect of the sigmoid colon on barium enema studies.