- •
In HF patients, CAD may be causal, contributory, or coincidental. The determination of the role of CAD in the patient’s cardiomyopathy is essential to determine the potential additive role of revascularization or device therapy over optimal medical therapy.
- •
The assessment of ischemia and viability helps differentiate when CAD is causal, contributory, or coincidental in a patient with HF and reduced LVEF.
- •
The assessment of stress-induced ischemia should be a key part of the evaluation of a patient with suspected ischemic cardiomyopathy and routinely performed if there are no clinical contraindications or safety concerns.
- •
There is no clear ideal noninvasive test for all clinical scenarios, and all approaches have strengths and weaknesses.
- •
SPECT and PET imaging have been central to the management of IHF and have reasonable accuracy to predict functional and clinical recovery after revascularization.
- •
Quantification of myocardial blood flow with PET provides an additional powerful tool to help define the contribution of ischemia to the patient’s cardiomyopathy.
- •
In some patients, multimodality imaging provides important complementary information for identification of potential revascularization candidates.
- •
The available evidence suggest that imaging of ischemia and viability may not be essential in patients with severe angina, adequate coronary targets for revascularization, and reasonable perioperative risk. Nevertheless, noninvasive imaging is a helpful aid for the determination of risk versus potential benefit and to define management strategies in higher-risk individuals.
- •
The role of multivessel PCI versus CABG in patients with IHF requires additional research.
Introduction
Nuclear cardiology imaging techniques are frequently employed to guide clinical decision making in patients with ischemic heart failure (IHF). Perhaps surprisingly, it is not always straightforward to distinguish whether coronary artery disease (CAD) is indeed causal, coincidental, or a contributor to IHF. Diagnosing the cause of heart failure (HF) is an important step to determining the management strategy, potential urgency, and need for revascularization. Nuclear cardiology—single photon computed tomography (SPECT) and cardiac positron emission tomography (PET)—applications can help determine the presence of ischemia, scar, hibernating myocardium, or sympathetic denervation abnormalities, all of which may help both in diagnosis, prognostication, and prediction of a response to therapy. Despite the clinical observation that cardiac PET and SPECT are useful imaging tools in IHF, clinical evidence to support their use has not been consistent. Although the routine use of viability imaging has been questioned in some clinical studies, there may be specific clinical scenarios where viability and ischemia imaging may aid in decision making. In this chapter, we will consider how ischemia and viability imaging remain valuable techniques to identify those patients in whom CAD is the cause or major contributor to their HF and how this can help in decisions for revascularization therapies. We will consider some of the foundational evidence of the importance of imaging in this area and highlight more recent controversial evidence, some of which supports and some of which is ambivalent toward their utility. We will also provide case vignettes to illustrate graphically how ischemia and viability imaging is used in this patient demographic.
Ischemic heart failure
The burden of ischemic heart disease (IHD) has increased within the past 30 years. Despite the fact that the prevalence of fatal myocardial infarction (MI) has decreased since the 1990s, there is a higher prevalence of IHD. This has been attributed to improvements in the treatment of acute coronary syndrome and stable coronary disease in combination with the increasing aging of the population. IHD is the main cause of HF, a major public health problem with a growing estimated prevalence of over 37 million cases worldwide. , HF affects up to 2% of the adult population in the developed world, with estimates of increasing prevalence over time. By 2030, the prevalence of HF is estimated to increase by 46% in the United States. The burden to patients and providers is significant, with an estimated annual cost of treatment exceeding $30 billion in the United States alone, equivalent to more than 10% of the total cardiovascular disease health expenditure, half of which is attributed to hospitalization. Interestingly, the increase in prevalence contrasts with a decline in incidence worldwide, partially attributed to advances in therapy and improvement in diagnostic technologies. In the United States, however, there are still 915,000 new cases of HF per year, corresponding to an incidence of 10 per 1000 subjects after 65 years of age. ,
Myocardial ischemia results from an imbalance between oxygen supply and demand. It can manifest as an acute coronary syndrome (ACS) or chronic IHD. The severity of inadequate flow will determine myocardial adaptations and the extent of reversibility once flow is restored. In patients with IHD, persistent ventricular dysfunction can result from repeated episodes of stress-induced ischemia with postischemic stunning (repetitive stunning), myocardial hibernation, or scar. Such persistent dysfunctional myocardium is considered viable when the ischemia-related injury (repetitive stunning or hibernation) is not severe or prolonged enough to cause cell death. In this case, cardiomyocyte dysfunction can be reversed (or partly reversed) if flow is restored with revascularization. Prolonged ischemic-related injury results in cell death with attendant scar tissue formation that replaces healthy myocardium. The recovery of is chemically injured myocardium that contains scar depends on the transmural extent of infarcted myocardium ( Table 20.1 ).
| Myocardium | Flow/Perfusion | Glucose Metabolism (FDG) | Function/Contractile Reserve | Structural Changes | Potential to Recover/Clinical Relevance |
|---|---|---|---|---|---|
| Viable | |||||
| Stunning | Preserved at rest (after transient ischemic insult) | Viable (normal, increased, or reduced) | Reduced | No | Likely to recover if ischemic injury does not persist or become repetitive; revascularization can prevent recurrent stunning |
| Hibernation | Reduced | Preserved or increased (= perfusion-metabolism mismatch) | Reduced | Yes, some | May have partial/delayed or full recovery if adequate revascularization achieved |
| Nonviable | |||||
| Scar | Reduced | Reduced | Absent | Fibrosis | Unlikely to recover with or without revascularization |
Myocardial ischemia, viability, and scar can be identified and quantified with different noninvasive tests, such as echocardiography, cardiac magnetic resonance (CMR), and radionuclide imaging. Each can evaluate different aspects of cellular and myocardial physiology, including perfusion, contractile reserve, cell membrane and mitochondrial integrity, cell metabolism, and fibrosis.
Despite the fact that assessment of ischemia and/or viability in patients with CAD and HF seems to be important, at least one recent report suggests that the use of these tests may be underutilized.
Principles of management
The treatment landscape for ischemic HF has changed considerably over the past 30 years and especially in the past decade, thereby contributing to significant reductions in morbidity and mortality. This includes advances in medical management and revascularization approaches. Indeed, the 12-month mortality has been reduced from over 50% to 10%. Optimal guideline-directed medical therapy remains the cornerstone of management for patients with HF, including those with CAD. In selected patients, the addition of high-risk revascularization improves clinical outcomes. , In this chapter, we will review the utility of noninvasive imaging for assisting in decisions regarding optimal patient management, especially the decision of when revascularization and potentially device therapy can help improve functional outcomes or prognosis.
Imaging techniques for the evaluation of ischemia and viability
IHF represents the impairment in contractility of (principally) the left ventricle (LV) due to obstructive CAD. Whether revascularization will improve contractility and/or outcome is determined by the balance of myocardial ischemia and scar to viable cardiomyocytes as well as other factors discussed later in this chapter. Unless stress testing is deemed too high of a risk because of the patient clinical status or coronary anatomy (e.g., critical left main or three-vessel CAD), it is generally reasonable to first assess the presence and magnitude of stress-induced ischemia. Please refer to Chapter 4 for a detailed discussion of radionuclide imaging techniques and protocols for stress radionuclide imaging. In patients without significant inducible ischemia and large apparent areas of scarring, there is a need to determine whether these regions may, in fact, contain significant viable myocardium. Methods to assess viability have nevertheless been established with echocardiography, SPECT or PET imaging, and cardiac magnetic resonance imaging (CMR).
Echocardiography
Echocardiography uses functional and morphologic appearances to help determine the presence of predominant scar versus viable myocardium ( Table 20.2 ). , LV end-diastolic wall thickness less than 6 mm on echocardiography is associated with scar as is a lack of response to dobutamine stress in segments with impaired wall motion at rest. Classically, patients with ischemic LV impairment demonstrate a biphasic response to dobutamine stress in viable segments with an initial improvement in segment function, but with increasing dobutamine doses the initial improvement typically deteriorates.
| Imaging Modality | Imaging Target | Indicator of Viability |
|---|---|---|
| 18 FDG PET | Glucose metabolism |
|
| SPECT ( 201 Tl) | Myocardial perfusion/Na/K ATPase activity (membrane integrity) |
|
| SPECT ( 99m Tc-based) | Myocardial perfusion/arterioles vasodilatation Mitochondrial integrity |
|
| Dobutamine echocardiography/MRI | Contractile reserve | Improvement in wall motion with low-dose dobutamine |
| Delayed enhancement MRI | Fibrosis tissue | Absence or small amount of scar |
| Late enhancement CT | Fibrosis tissue | Absence or small amount of scar |
| Myocardial contrast echocardiography | Microvascular integrity | Homogeneous contrast intensity |
Cardiac magnetic resonance imaging
Segments with wall thicknesses of 5 mm or less are considered nonviable. A key technique to assess the presence of scars with CMR includes the use of the burden of gadolinium contrast enhancement on delayed imaging (so-called late gadolinium enhancement [LGE]). LV segments with equal to or greater than 50% of their transmural thickness showing LGE are thought to be mostly scar and nonviable. The magnitude of stress-induced ischemia is usually assessed using first-pass perfusion. Like with echocardiography, the wall motion response to dobutamine stress can also be used to assess ischemic burden.
Radionuclide imaging approaches
SPECT imaging
201 Thallium (Tl) and 99m technetium (Tc)-labeled tracers can be used to evaluate myocardial viability versus scar based on the severity of perfusion defects (see Table 20.2 ). As discussed in Chapter 4 , both radiotracers are actively taken up by viable cardiomyocytes and can be used with rest-only and rest-stress protocols as indicators of viable and/or ischemic myocardium.
For rest-stress protocols, the presence of inducible myocardial ischemia in dysfunctional segments reflects viable but jeopardized myocardium. For rest-only protocols, myocardial segments with greater than 50% of peak radiotracer uptake in the LV are considered to be viable. In addition, a regional increase in radiotracer uptake greater than 10% (compared with baseline) in the 4- or 24-hour delayed (redistribution) 201 Tl images or postnitrate administration, most commonly used with 99m Tc-labeled tracers, are also indicators of myocardial viability. The extent of dysfunctional but viable segments is then summed to determine the likelihood of LV ejection fraction (LVEF) improvement after revascularization. In general, the larger the amount of dysfunctional but viable myocardium, the higher the likelihood of improvement in LVEF after revascularization. This concept applies to all imaging methods. Nevertheless, the optimal quantitative threshold of myocardial viability to predict improvement in LVEF after revascularization is reportedly different across imaging methods. Another practical way to predict the likelihood of improvement in LV function after revascularization is to consider the volume of the myocardial scar in the LV. For example, in the P ositron emission tomography A nd R ecovery following R evascularization (PARR-1) study, little improvement in global LV function with revascularization was observed in patients with myocardial scar volume involving equal to or greater than 27.5% of the LV, which is in keeping with findings from cardiac MRI. Conversely, patients with less than 16% scar volume had significant LVEF improvement after revascularization. Functional recovery also depends on other factors, including the degree of LV remodeling and the timing and completeness of revascularization.
PET imaging
The PET viability protocol includes an assessment of myocardial perfusion at rest and stress, when clinically appropriate (see protocols in Chapter 4 ), followed by a metabolic assessment with 18 F-fluorodeoxyglucose (FDG). As discussed in Chapter 19 , in the glucose-loaded state and in ischemia (regardless of glucose loading), glucose is the preferred substrate for myocardial energy metabolism. Under these circumstances, FDG uptake and retention reflects the rate of exogenous glucose utilization and is a marker of myocardial viability. In a meta-analysis of viability imaging trials, the radionuclide methods, especially FDG PET, were the most sensitive techniques for detecting viable myocardium.
Patient preparation for FDG imaging
Because utilization of energy-producing substrates by the myocardium is largely a function of their concentration in plasma and hormone levels (especially plasma insulin, insulin/glucagon ratio, growth hormone, and catecholamines) and of oxygen availability for oxidative metabolism, careful patient preparation is necessary to obtain diagnostic FDG images. For a detailed step-by-step description of the available methods for patient preparation before FDG imaging, the reader should review the “Guidelines for PET Imaging” published by the American Society of Nuclear Cardiology and the Society of Nuclear Medicine. Briefly, the available approaches to patient preparation include:
Fasting
Fasting is the simplest method because it does not require any substrate manipulation. With this approach, ischemic but viable tissue shows as a “hot spot” because of the preferential free fatty acid (FFA) utilization by normal (nonischemic) myocardium. Although imaging interpretation would seem straightforward, the lack of tracer uptake in normal (reference) myocardium may occasionally lead to an overestimation of the amount of residual viability within a dysfunctional territory.
Oral or intravenous glucose loading
This is the recommended approach to FDG imaging. The goal of glucose loading is to stimulate the release of endogenous insulin to decrease the plasma levels of FFA and facilitate the transport of FDG into cardiomyocytes. Patients are usually fasted for at least 6 hours and then receive an oral or IV glucose load. Most patients require the administration of IV insulin to maximize myocardial FDG uptake.
Hyperinsulinemic-euglycemic clamp
This approach is technically demanding and time-consuming. It consists of a constant infusion of insulin IV with adjustments in glucose infusion to avoid hypoglycemia until the body reaches steady-state between glucose infusion and disposal. At this point, no further adjustments are necessary and FDG can be administered. Because it is technically demanding, most laboratories reserve this approach for challenging conditions (e.g., diabetes and severe congestive HF).
Free fatty acid inhibition
Acipimox (not available in the United States) and niacin are both nicotinic acid derivatives that inhibit peripheral lipolysis, thereby reducing plasma FFA levels and, indirectly, forcing a switch to preferential myocardial glucose utilization. These drugs are usually given 60 to 90 minutes before FDG administration.
Dysfunctional myocardial segments can demonstrate the following perfusion and FDG viability patterns ( Fig. 20.1 ) :
- a.
Normal perfusion and metabolism: This refers to dysfunctional segments with normal or near-normal perfusion and FDG uptake. These segments may demonstrate evidence of stress-induced ischemia and are considered viable.
- b.
Perfusion-metabolism match: This refers to dysfunctional segments showing a concordant reduction in both perfusion and FDG uptake. It is indicative of transmural or nontransmural scar and nonviable myocardium.
- c.
Perfusion-metabolism mismatch: This refers to dysfunctional segments showing reduced perfusion with relatively preserved FDG uptake and represents viable but hibernating myocardium .
- d.
Perfusion-metabolism reverse mismatch: This refers to dysfunctional segments showing normal or near-normal perfusion with reduced FDG uptake. It may be seen in patients with diabetes where glucose/FDG uptake is suboptimal because of insulin resistance or low insulin levels, in those with left bundle branch block (LBBB), or in the setting of postischemic stunning. Regardless of the mechanism or clinical scenario, the presence of normal or near-normal perfusion always indicates myocardial viability. ,

In LV segments with reduced perfusion, the presence of FDG retention of greater than 50% of peak LV counts indicates the presence of myocardial viability, whereas FDG uptake values less than 50% are associated with predominantly nonviable myocardium. , In addition, using this model, patients with mismatch/hibernating myocardium equal to or greater than 7% had improved prognosis with revascularization. Similar improvement in prognosis was seen in 648 consecutive patients undergoing 18 F-FDG/ 82 rubidium (Rb) PET when revascularization was performed in patients with greater than 10% hibernating myocardium ( Fig. 20.2 ).

Patient-centered imaging applications in ischemic heart failure
Case vignette 1
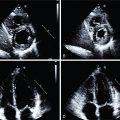
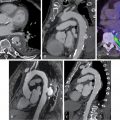
A 62-year-old female with diabetes presented with New York Heart Association (NYHA) Class III to IV HF symptoms. Echocardiography revealed a normal LV size with an LVEF of 28% and severe global hypokinesis without significant valvular disease. Follow-up invasive coronary angiography demonstrated an occluded proximal codominant right coronary artery (RCA), severe distal left main (LM) stenosis (70%), occluded mid left anterior descending (LAD) artery, and severe distal left circumflex (LCx) stenosis (90%) ( Fig. 20.3 ).

In view of the complexity of CAD and severe LV dysfunction, the patient underwent a rest myocardial perfusion and FDG PET scan to evaluate myocardial viability ( Fig. 20.4 ). The PET scan demonstrated a large and severe resting perfusion defect involving the mid anterior and anteroseptal walls, the apical LV segments, and the LV apex, showing moderate increased FDG uptake (partial perfusion-FDG mismatch, implying a mixture of nonviable and viable myocardium). There was also a medium-sized and severe perfusion defect throughout the inferior and inferoseptal walls, with normal FDG uptake (perfusion-FDG mismatch). Semiquantitative analysis confirmed a small area of nontransmural scar involving 8.8% of the LV mass in the mid LAD territory, and a large area of hibernating myocardium involving 21% of the LV mass in the LAD and RCA territories. Resting myocardial perfusion was normal in the LCx territory (see Fig. 20.4 ).

Despite the severity and extent of CAD and LV dysfunction, this patient’s PET scan showed a relatively small amount of scar with extensive areas of viable myocardium in all coronary territories, including a large area of hibernating myocardium in the LAD and RCA territories. Coronary revascularization was recommended on the basis of the coronary anatomy and PET findings. The patient underwent coronary artery bypass grafting (CABG) with left internal mammary artery (LIMA) graft to LAD, and saphenous vein grafts (SVGs) to the obtuse marginal (OM1) and posterolateral branches of the LCx artery and posterior descending artery branch of the RCA. Three months post CABG, her LVEF improved to 44%.
Patients with advanced HF should always be assessed for potential reversible causes of HF. If ischemia is suspected, revascularization should be considered and noninvasive imaging of ischemic and scar burden may be of benefit. The detailed quantitative information regarding the extent of scar, viability, and LV function provided by noninvasive imaging studies can play a key role in clinical decision making. The role of myocardial viability testing may not be essential in patients with severe ischemia or angina, good target vessels for revascularization, and minimal comorbidities. Nevertheless, ischemia/viability testing is helpful in high-risk patients, including elderly patients without angina, severe LV remodeling, prior CABG, and/or significant comorbidities. In such cases, the viability information can provide some guidance regarding the potential benefit from revascularization and help in decision making ( Table 20.3 ). Decisions regarding revascularization are complex in these patients and cannot be based solely on a single patient characteristic or imaging test. For example, although it may seem intuitive to suggest that anginal symptoms may predict benefit from revascularization, the presence or absence of angina alone should not be used as a discriminating factor to decide for or against revascularization. Indeed, a substudy of the Surgical Treatment for IschemiC Heart failure (STICH) trial demonstrated that angina did not predict mortality in patients with known CAD treated medically and also did not identify patients with LV dysfunction and CAD who have a greater benefit from CABG. Thus the integration of the patient profile with the imaging study may aid in decision making in patients in whom there is the most uncertainty about whether to proceed with revascularization ( Fig. 20.5 ).