- •
Radionuclide and multimodality imaging are essential to the care of patients at risk for incident cardiovascular disease during or after cancer treatment, either because of preexisting cardiovascular risk factors or because of the side effect profile of traditional or novel therapies; patients with primary or metastatic tumors involving the heart; and long-term cancer survivors requiring periodic evaluation for cardiovascular disease that may or may not be related to their previous cancer therapy.
- •
Planar and SPECT MUGA imaging techniques are accurate and reproducible for the evaluation of cardiac function before, during, and after cancer treatment. Echocardiography and CMR imaging are preferred for this purpose because of their lower radiation exposure.
- •
CAD is a preexisting diagnosis in many patients with newly diagnosed cancer and has increased in prevalence as cancer patients survive longer. Radionuclide MPI can be used effectively in the evaluation of CAD. Stress echocardiography, stress cardiac MRI, and CCTA also have roles in the assessment of CAD in patients with cancer and in cancer survivors. Additionally, PET can assess for the presence of CMD via myocardial blood flow assessment, and CT can be used for CAC scoring.
- •
Multimodality cardiovascular imaging plays an important role in the diagnosis and management of cardiac neoplasms, which are rare. In addition to echocardiography, which is usually a first-line test, metabolic PET/CT and CMR are often used to make the diagnosis and better understand the location and extent of involvement.
- •
An emerging application of radionuclide imaging in cardio-oncology is metabolic PET imaging for the evaluation of ICI-related toxicity, including myocarditis. CMR imaging and GLS assessment via echocardiography are also currently being studied in this entity.
Introduction
The field of cardio-oncology is an emerging discipline that aims to provide comprehensive and multidisciplinary cardiovascular care to patients with cancer. Cardio-oncology programs across the world aim to provide cardiovascular care for patients during cancer treatment and posttreatment during cancer survivorship. These include the following groups: (1) patients with preexisting cardiovascular disease or risk factors who have newly diagnosed cancer and need to be safely guided through medical, surgical, and radiation treatment for their cancer; (2) patients who are at risk for incident cardiovascular disease during or after treatment because of the side effect profile of traditional or novel cancer therapies; (3) patients with a primary tumor of the heart or metastatic disease to the heart; and (4) long-term cancer survivors who need to be regularly evaluated for cardiovascular disease that may or may not be related to their previous cancer therapy.
The incidence and prognosis of cardiotoxicity from cancer therapy can vary depending on the age at treatment, sex, agent(s) used, cumulative dose, concomitant treatment with other cardiotoxic therapies, and other factors, including underlying cardiovascular risk. A retrospective cohort study of 14,358 5-year survivors of childhood cancer found that they were approximately five times more likely to report congestive heart failure (HF), myocardial infarction (MI), pericardial disease, or valvular abnormalities than their cancer-free siblings. A prospective, multicenter registry that studied 865 adult patients receiving potentially cardiotoxic cancer therapies found that cardiotoxicity (defined as new or worsening myocardial damage/ventricular function) was identified in 37.5% of patients over a median follow-up time of 24 months. The two most well-known agents associated with cardiotoxicity are anthracyclines and trastuzumab. Newer therapies, such as certain tyrosine kinase inhibitors and certain forms of immunotherapy, can also cause cardiotoxicity. The total lifetime cumulative dose of anthracycline received is the most important determinant of cardiotoxicity risk. The highest risk for cardiotoxicity with trastuzumab therapy is when it is administered after anthracycline therapy: as many as 27% of patients can develop a significant decline in left ventricular (LV) function and as many as 4% of patients can develop symptomatic HF.
There are, however, several other forms of cardiotoxicity associated with cancer therapy besides ventricular dysfunction, including coronary artery disease (CAD), hypertension, myocarditis, valvular heart disease, pericardial disease, venous thromboembolic disease, arrhythmias, and conduction system abnormalities. Additionally, neoplasms can involve the heart as primary cardiac tumors or as a site of metastases. Radionuclide imaging techniques and multimodality cardiovascular imaging are essential to the field of cardio-oncology. In this chapter, we review current key uses of radionuclide imaging (and other modalities) in cardio-oncology and describe emerging applications. Cardiac amyloidosis is discussed separately in Chapter 24 .
Patient-centered applications of multimodality imaging in cardio-oncology
Case vignette 1: Evaluation of cardiac function
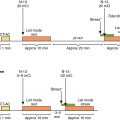
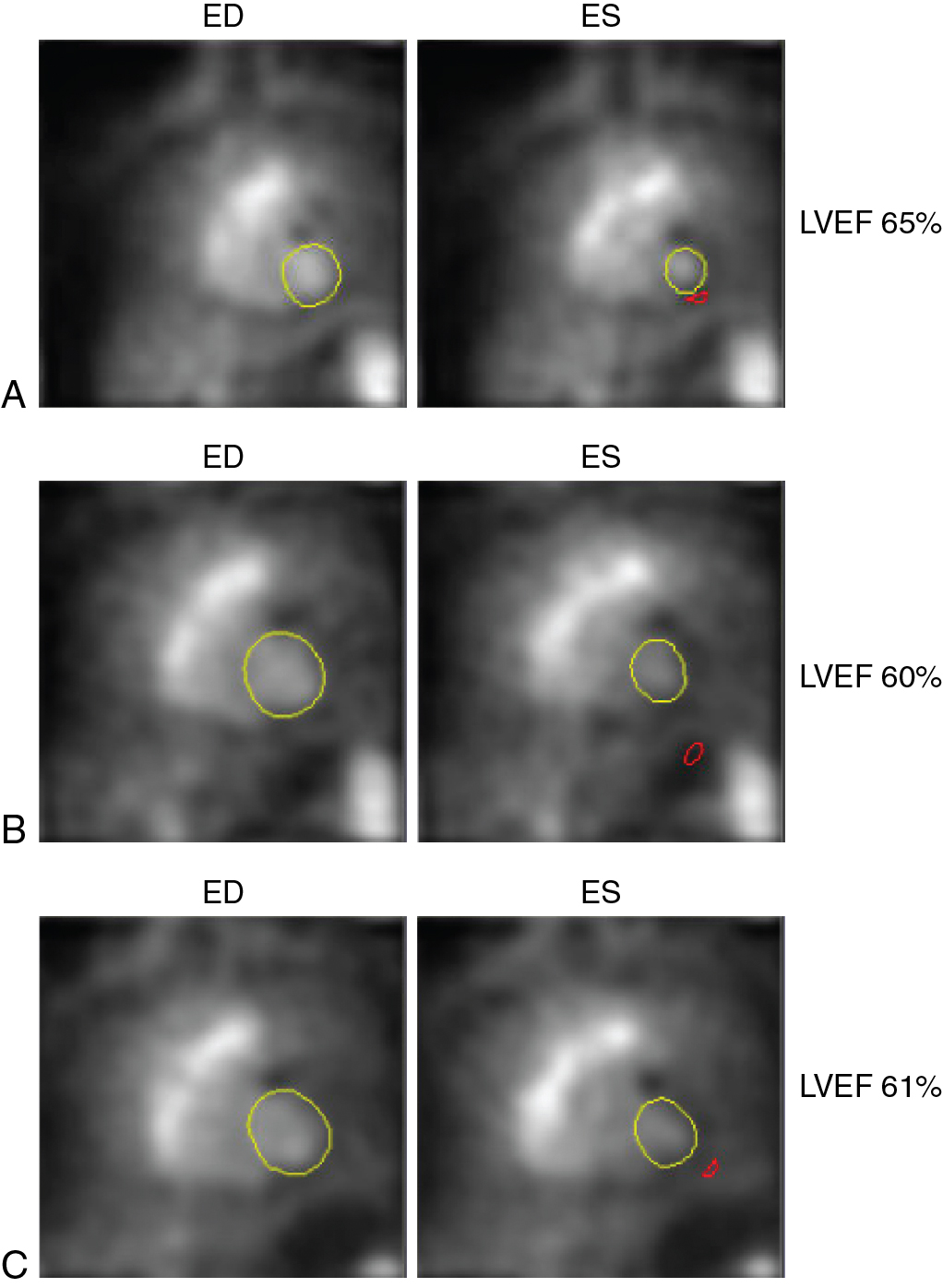
A 33-year-old female presented to her primary care physician with a right breast lump. She was referred for an ultrasound and a mammogram, and both were suspicious for malignancy. Biopsy confirmed estrogen receptor, progesterone receptor, and HER2-positive breast cancer. She underwent bilateral mastectomies. Baseline cardiac function was obtained via 99m technetium (Tc) red blood cell radionuclide ventriculography (also known as multigated acquisition [MUGA]), and her estimated LV ejection fraction (LVEF) was 65%. She was treated with four cycles of doxorubicin and cyclophosphamide and underwent repeat radionuclide ventriculography, which revealed an LVEF of 60%. She then received 12 weeks of paclitaxel and trastuzumab. Repeat radionuclide ventriculography revealed an LVEF of 61% ( Fig. 25.1 ).
The American Society of Clinical Oncology states that the following patients with cancer are at increased risk for developing cardiac dysfunction:
- •
those receiving high-dose anthracycline therapy (e.g., doxorubicin ≥250 mg/m 2 ),
- •
those receiving concomitant high-dose radiation therapy (≥30 Gy) where the heart is in the treatment field,
- •
those receiving lower-dose anthracycline therapy in combination with lower-dose radiation therapy where the heart is in the field,
- •
those receiving lower-dose anthracycline or trastuzumab therapy alone and who have any of the following risk factors: at least two cardiovascular risk factors, age equal to or greater than 60, or known cardiovascular disease, and
- •
those receiving treatment with lower-dose anthracycline followed by trasutuzmab.
The guideline recommends assessment of LVEF at baseline in all patients who meet criteria for increased risk with reassessment within 1 year of completing anthracycline therapy. The guideline recommends discontinuation of doxorubicin if there is an absolute decrease in LVEF of at least 10% from baseline to no more than or equal to 50%. The package insert for trastuzumab recommends monitoring cardiac function at baseline and every 3 months during trastuzumab treatment. The European Society for Medical Oncology Clinical Practice Guidelines recommend that if an asymptomatic patient’s LVEF drops 10% below baseline to less than 50%, trastuzumab should be held and LVEF should be reassessed in 3 weeks. Angiotensin-converting enzyme (ACE) inhibitor and beta-blocker therapy should be initiated in asymptomatic individuals with an LVEF of less than 40% and should be considered in individuals, like this patient, with a decline in EF greater than 10% from baseline to an LVEF of less than 50%. If the LVEF is less than 40% on repeat assessment, trastuzumab therapy should be discontinued. If the repeat LVEF is at least 40%, the patient can be rechallenged with trastuzumab with frequent monitoring for a recurrent decline in LV function.
In the following sections, we discuss radionuclide imaging options for evaluation of cardiac function before, during, and after treatment (planar or single-photon emission computed tomography [SPECT] MUGA imaging, echocardiography, and cardiac magnetic resonance [CMR] imaging).
Multigated acquisition imaging
MUGA imaging (also known as radionuclide angiography, equilibrium radionuclide angiography, radionuclide ventriculography, and gated blood pool scan) is used to determine global and regional measures of ventricular function. The technique consists of radiolabeling the patient’s own RBCs, which are then reinjected for imaging. For a full description of the technique, please refer to the American Society of Nuclear Cardiology guidelines. Briefly, there are two methods for labeling the RBCs: (1) using in vivo or modified in vivo/in vitro methods (e.g., using 2 to 3 mg stannous pyrophosphate 15 minutes before injection of 99m Tc), or (2) using a commercial in vitro kit, which is the more common option. Radiolabeled blood cells are then reinjected and, after 1 to 2 minutes, electrocardiogram (ECG)-gated equilibrium blood pool imaging is obtained with planar or tomographic (SPECT) imaging. End-diastolic and end-systolic volumes are then measured to calculate the LVEF.
An anthracycline-associated reduction in LV systolic function was first observed in the 1970s via MUGA imaging. In a study published from Yale University School of Medicine in 1979, MUGA imaging was used to estimate LVEF in 55 patients receiving doxorubicin therapy. The investigators found that all five patients who had clinical HF had an ejection fraction of less than 30% by quantitative MUGA imaging at the time of symptom development. Additionally, six patients who had moderate toxicity, which involves a decline in ejection fraction by at least 15% to a final value of less than 45%, in whom doxorubicin was discontinued, did not have clinical HF or a further decline in ejection fraction during the follow-up period. Therefore the authors concluded that “assessment of radionuclide LVEF during doxorubicin therapy may make it possible to avoid congestive heart failure.” A follow-up study that monitored 1487 patients with cancer over a 7-year period with serial resting MUGA imaging developed criteria for monitoring LVEF in patients receiving doxorubicin and for deciding when to discontinue doxorubicin therapy. The occurrence of symptomatic HF was compared in patients followed according to these criteria and in patients who were not. They found that the former group had a significantly lower incidence of symptomatic HF (2.9% vs. 20.8%), and there were no deaths from HF in that group.
Single photon emission computed tomography
ECG-gated SPECT MUGA was developed more recently and has some advantages over traditional planar MUGA imaging. Gated SPECT MUGA has been shown to provide accurate measures of LVEF, right ventricular (RV) ejection fraction, and LV and RV end-systolic and end-diastolic volumes via automatic geometric methods. Moreover, LV volume indices and LVEF measured by SPECT MUGA are predictive of cardiovascular events. Nevertheless, the prognostic value of SPECT MUGA in cardio-oncology has yet to be studied. Finally, newer, solid-state, cadmium zinc telluride SPECT cameras provide greater sensitivity and better spatial resolution and can provide high-quality images with low doses of radiation (∼10 mSv) and shorter scan times (see Chapter 1 ). These features make it an attractive option for assessing ventricular function in a patient with a history of cancer.
Myocardial perfusion imaging (MPI) via SPECT and cardiac positron emission tomography (PET) imaging can also be used to obtain accurate LVEF assessments (see Chapters 1 and 2 ), and their use in patients undergoing cancer treatment will be discussed later in this chapter.
Echocardiography
Over time, echocardiography has become the modality of choice for baseline and serial LVEF assessment in patients with a history of malignancy. Unlike radionuclide MUGA (average effective radiation dose: ∼10 mSv), echocardiography uses ultrasound and does not involve radiation. This is an advantage in imaging patients with cancer who may have a history of radiation therapy or who may be candidates for future treatment with radiation and for whom reducing cumulative radiation exposure is important. Echocardiography can also provide valuable information regarding the presence or absence of valvular heart disease, as well as hemodynamic measures such as estimated RV systolic and left atrial filling pressures.
Although the modality is commonly used in cardio-oncology, there are important limitations to echocardiography that should be considered on a case-by-case basis. Most importantly, poor overall image quality can severely limit its ability to accurately estimate LVEF and can lead to reduced reproducibility. This can occur because of a lack of suitable windows for the ultrasound probe on the patient’s body; surgical changes on the patient’s body that prohibit the placement of the ultrasound probe (e.g., chest bandages after a mastectomy); prosthetics, such as breast implants; morbid obesity; or severe obstructive lung disease (as can be associated with lung cancer in some patients). Poor endocardial border delineation can also be problematic, especially if contrast agents are not available to opacify the LV blood pool. Planar or SPECT MUGA or CMR should be considered in these cases. Finally, planar and SPECT MUGA have high accuracy, high reproducibility, and have a lower interobserver variability (<5%) than has been reported for two-dimensional echocardiography in adults (e.g., 14% ).
Global longitudinal strain
Strain imaging of the LV via echocardiography is increasingly used to evaluate cardiac deformation and function. Vendor-specific algorithms are used to obtain longitudinal strain values from apical echocardiographic images either at the time of image acquisition or during postprocessing. Longitudinal strain reflects the change in the distance between two segments of the myocardium relative to the distance at baseline.
Recent studies have shown that strain imaging can detect cardiotoxicity before reductions in LVEF and may therefore be used as a prediction imaging biomarker. In a study involving 43 patients with breast cancer who received anthracyclines and trastuzumab, 9 developed cardiotoxicity. A decrease in longitudinal strain from baseline to 3 months was an independent predictor of the development of cardiotoxicity at 6 months (15 ± 8% in the cardiotoxicity present group vs. 3 ± 10% in the cardiotoxicity absent group). In a study of 81 women with newly diagnosed HER2-positive breast cancers who were treated with anthracyclines followed by taxanes and trastuzumab, surveillance echocardiography was performed every 3 months. Twenty-six patients developed cardiotoxicity, and peak systolic longitudinal myocardial strain measured at the completion of anthracycline therapy predicted the development of subsequent cardiotoxicity. Global longitudinal strain (GLS) was less than 19% in all patients who later developed symptomatic HF. Nevertheless, current guidelines do not recommend cardioprotective therapy for patients with asymptomatic reductions in GLS because of a lack of sufficient evidence linking abnormalities in strain imaging with HF. The ongoing randomized controlled Strain sUrveillance of Chemotherapy for improving Cardiovascular Outcomes (SUCCOUR) trial aims to evaluate the “hypothesis that GLS guidance of cardioprotective therapy [will] improve cardiac function of at-risk patients undergoing potentially cardiotoxic chemotherapy, compared with usual care.”
Cardiac magnetic resonance imaging
CMR imaging is also used to assess LV function before, during, and after cancer therapy and, like echocardiography, does not use ionizing radiation. LVEF is quantified via CMR, most frequently using a series of contiguous short-axis slices from the cardiac apex to the base using a steady-state cine imaging technique. End-diastolic and end-systolic LV cavity areas are obtained from each slice, and volumes are obtained by multiplying them by the slice thickness. The volumes are added up and then LVEF is calculated by subtracting the end-systolic volume from the end-diastolic volume. Advantages of CMR imaging for evaluation of cardiac function include high spatial and temporal resolution, reproducibility, and accuracy for LVEF quantification to detect subclinical declines in LVEF. A study that included 22 patients before and after anthracycline chemotherapy found that CMR was able to detect a reduction in LVEF after just 28 days of therapy.
Although CMR has many advantages due to its high image quality, precision, and reproducibility, there are important disadvantages worthy of consideration. CMR imaging is costlier than planar MUGA imaging and echocardiography, is not as widely available as echocardiography, and has important patient-related contraindications, including claustrophobia and the presence of metallic implants that are not magnetic resonance imaging (MRI)–compatible.
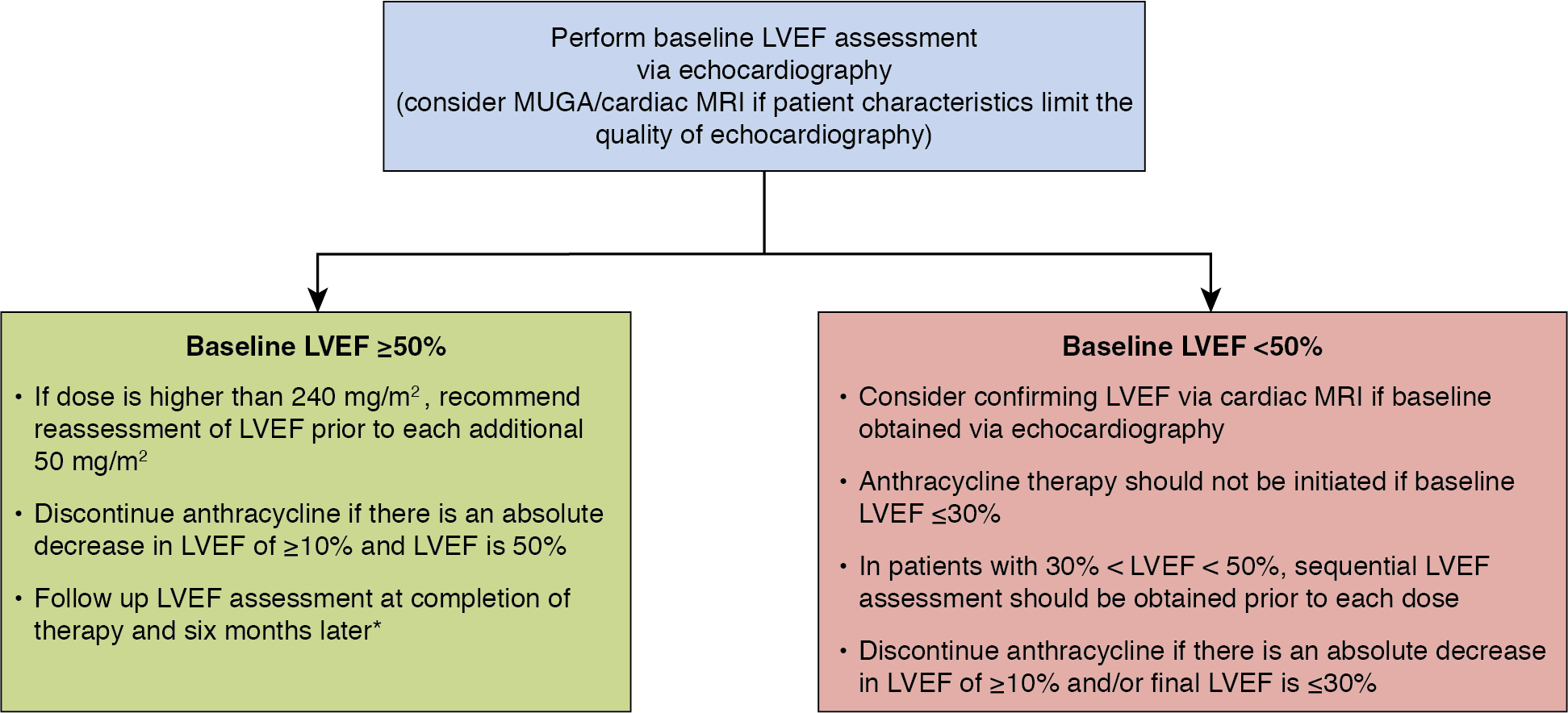
Table 25.1 summarizes and compares the modalities for cardiac function assessment previously discussed, and Fig. 25.2 outlines suggestions for monitoring patients receiving anthracyclines. ,
| Modality | Advantages | Limitations |
|---|---|---|
| Multigated acquisition imaging (MUGA) | Highly reproducible and accurate | Involves radiation exposure |
| Highly available | Cannot obtain data on valvular function, hemodynamic data, etc. | |
| Single-photon emission computed tomography (SPECT) MUGA | Highly reproducible | Involves radiation exposure |
| Spect myocardial perfusion imaging (MPI) | Highly available Concurrent assessment for coronary artery disease | Involves radiation exposure |
| Positron emission tomography (PET)/computed tomography (CT) MPI | Concurrent assessment for coronary artery disease | Involves radiation exposure Less available |
| Echocardiography | Does not involve ionizing radiation Lower cost | Image quality limited by patient characteristics such as obesity, lung disease, adequate imaging windows, etc. |
| Highly available | Lower reproducibility and accuracy | |
| Can assess valvular function, hemodynamic data, global longitudinal strain, etc. | Interreader variability | |
| Cardiac magnetic resonance (CMR) imaging | Does not involve ionizing radiation | Less available |
| Highly reproducible and accurate | Higher cost | |
| Can assess valvular function but not as well as echocardiography | Patient-related relative contraindications (claustrophobia, metallic implants, etc.) | |
| Can assess myocardial edema and fibrosis |
Case vignette 2: Evaluation of epicardial coronary artery disease
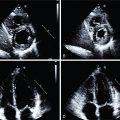
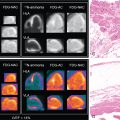
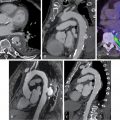
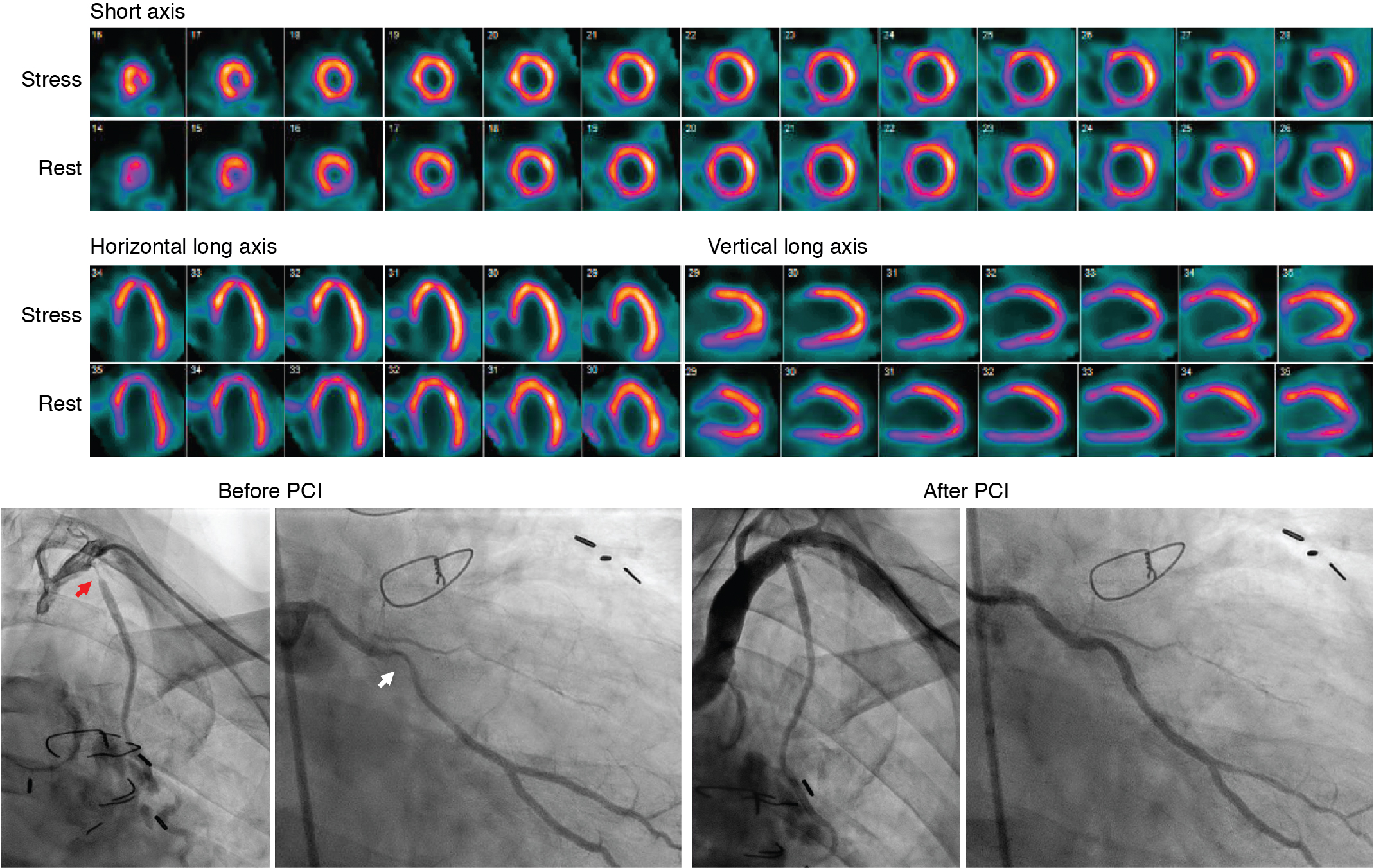
A 49-year-old male with a history of Hodgkin’s lymphoma diagnosed at age 17 and treated with nitrogen mustard and mantle/upper abdominal radiation was seen by his cardiologist for recurrent chest pain. He has a history of multiple complications of radiation therapy, including CAD requiring single-vessel coronary artery bypass graft surgery (left internal mammary artery [LIMA] to left anterior descending [LAD] artery) and subsequent percutaneous coronary intervention (PCI) to the left circumflex and right coronary arteries. He was referred for an exercise stress SPECT study because of chest pain to evaluate for progressive obstructive CAD. He exercised for 8 minutes and 30 seconds of a modified Bruce protocol. His heart rate decreased with exercise from 86 beats per minute (bpm) at rest to 80 bpm with exercise, and his blood pressure decreased from 132/80 mm Hg at rest to 110/70 mm Hg at peak exercise. Exercise was terminated because of shortness of breath and exercise-induced hypotension. The patient developed typical chest pain 6 minutes into the test that resolved 2 minutes into recovery. The ECG response to exercise was nondiagnostic. SPECT MPI revealed a small-sized severe perfusion defect involving the basal anteroseptal and anterior walls that was significantly but not completely reversible ( Fig. 25.3 ). Coronary angiography revealed severe multivessel CAD and a severe stenosis at the ostium of the LIMA graft. After discussion with a multidisciplinary heart and vascular team, he underwent successful PCI to the LIMA and an obtuse marginal branch (see Fig. 25.3 ).
CAD is a frequent comorbid illness in patients diagnosed with cancer, has increased in prevalence in cancer survivors as this population continues to live longer, and is a known form of cardiotoxicity of several cancer therapies. In particular, radiation therapy has been associated with an increased risk for obstructive CAD. , Because of this, guidelines recommend functional stress testing for the evaluation of obstructive CAD 5 to 10 years after exposure in high-risk patients, even if they are asymptomatic. We will discuss the evaluation of epicardial CAD in cardio-oncology in the following sections.
Myocardial perfusion imaging
MPI via SPECT or PET has an important role in the evaluation of patients with cancer and cancer survivors in several different clinical scenarios. These include survivorship surveillance for patients at risk for development of CAD due to their cancer therapy and patients without known CAD but with symptoms concerning for flow-limiting CAD and for the evaluation of patients with known CAD. In addition to rest and stress myocardial perfusion information, the use of exercise stress captures important data, including exercise capacity, the presence or absence of symptoms with exercise, and abnormal hemodynamic exercise responses that are associated with poor outcomes in the general population and in cancer survivors, as illustrated in Case Vignette 2.
Radiation therapy, where the heart is in the treatment field, remains the cancer therapeutic associated with the highest risk for the development of CAD, most commonly in the LAD coronary territory because of its anterior location. Common malignancies treated with chest radiation therapy include lymphoma, breast cancer, lung cancer, and esophageal cancer. The risk for radiation-associated CAD increases linearly with the mean radiation dose to the heart, with no apparent threshold. A case-control study of 2168 women in Sweden and Denmark found that the rates of major coronary events increased linearly by 7.4% for every gray increase in mean dose to the heart. The estimated incidence of radiation therapy–associated CAD was approximately 9% by 20 years posttherapy.
Several studies have shown the utility of SPECT MPI in the evaluation of patients postradiation therapy. A study of 23 patients with left-sided breast cancer treated with radiation therapy with and without doxorubicin found that 60% of patients had new visible perfusion defects 6 months postradiation therapy. A study of 69 patients treated with radiation therapy for left-sided breast cancer who underwent preradiation and postradiation therapy SPECT MPI found that there were increased perfusion defects in the LAD territory 6 months postradiation compared with preradiation therapy. These changes were independently associated with the volume of the LV irradiated, hormonal therapy, and the presence of baseline hypercholesterolemia. A retrospective study that included 961 patients with breast cancer treated at one center found that of the 27 patients with left-sided breast cancer who had abnormal stress tests, 70% of the abnormalities involved the LAD territory. A study that included 44 patients with lung cancer who received radiation therapy and 44 risk-matched control patients who underwent MPI found that defects in the lung cancer group were in the anterior and septal segments, whereas they were in different myocardial segments in the control group.
PET MPI is another radionuclide option for evaluation of known or suspected CAD in patients with a history of cancer. The availability and use of cardiac PET continue to increase and PET is now the gold standard for MPI because of its higher spatiotemporal resolution, sensitivity, and accuracy compared with SPECT. , Another key advantage of PET is its ability to quantify myocardial blood flow and flow reserve (see Chapter 3 ).
In addition to radiation therapy, several other cancer therapeutics are associated with an increased risk for CAD. The incidence of cardiotoxicity with 5-fluorouracil has been reported to be as high as 19%, although most studies report a risk of 7.6% or less. One of the cardiotoxicities associated with 5-fluorouracil is coronary vasospasm, which can present as chest pain, ECG changes, and/or MI. Many tyrosine kinase inhibitors affect the vascular endothelial growth factor pathway. Although hypertension is the most commonly associated cardiotoxicity with tyrosine kinase inhibitors, others include arterial thromboembolism, severe peripheral artery disease, cardiac ischemia, and MI. Additionally, the multitargeted tyrosine receptor kinase–inhibitor sunitinib has been associated with coronary microvascular dysfunction in a preclinical study, and nilotinib and ponatinib have been associated with acute vascular events. Treatment with platinum-based chemotherapy is associated with vascular toxicity and is associated with a long-term risk for CAD. MPI can be effectively used in patients with a history of treatment with these agents, and others that were not discussed here, to evaluate for the presence of CAD.
Other imaging approaches to coronary artery disease in cardio-oncology patients
In addition to radionuclide MPI, several other modalities can be used for the assessment of CAD in cardio-oncology. These include coronary computed tomography angiography (CCTA), exercise and pharmacologic stress echocardiography, and stress CMR imaging.
Coronary computed tomography angiography
CCTA uses an IV injection of contrast to opacify the coronary artery lumen, thereby allowing for anatomic assessment of both obstructive and nonobstructive atherosclerosis. CCTA has high-spatial resolution and a high sensitivity for calcium in the coronary arteries, which is associated with atherosclerosis. Additionally, almost all imaging acquisition protocols produce high-quality images and expose patients to less than 5 mSv of radiation. Emerging data from small studies support the selective use of CCTA in patients with cancer. In a study of 119 survivors of childhood Hodgkin’s lymphoma (mean age at cancer diagnosis was 8.3 years and mean age at the time of the study was 20 years), 19 patients (16%) had a coronary artery abnormality. A study of nine consecutive patients who had mediastinal radiation therapy for Hodgkin’s lymphoma found that eight of the nine patients had nonobstructive or obstructive CAD (mean age at cancer diagnosis was 19 years and mean age at the time of the study was 45.6 years). For patients who require surveillance for CAD because of a history of cardiotoxic cancer therapy but have a low pretest probability of obstructive CAD because of young age, low estimated cardiovascular risk, and exercise tolerance, CCTA may be a good option for testing because the discovery of nonobstructive CAD may present an opportunity for lifestyle- and medication-based risk factor modification.
Stress echocardiography
Because of widespread availability and the lack of radiation exposure, stress echocardiography is often the test of choice for evaluation of CAD in cancer survivors. Although a full resting study is not typically obtained before stress (via exercise or pharmacologic stress), significant valvular disease can be identified at the time of the study as well. Resting wall motion abnormalities can be a sign of myocardial injury, but a stress-induced wall motion abnormality is highly sensitive and specific for ischemia and obstructive CAD. A study that enrolled 294 patients after mediastinal radiation of at least 35 Gy for Hodgkin’s disease and referred patients for both stress echocardiography and radionuclide MPI found that 21.4% had abnormal wall motion at rest and 14% developed reversible MPI defects, impaired wall motion, or both abnormalities (mean time after radiation was 15 ± 7 years). In a subset of patients who underwent coronary angiography, the positive predictive values for stress echocardiography for detecting an at least 50% and at least 75% stenosis were 87% and 80%, respectively. The positive predictive values for SPECT MPI for detecting an at least 50% and at least 75% stenosis were 45% and 31%, respectively. An important limitation of stress echocardiography is that the quality of stress images is highly dependent on the quality of the resting images. If the previously discussed limitations for echocardiography result in poor-quality resting images, an alternate modality should be considered to assess for CAD.
Stress cardiac magnetic resonance imaging
CMR imaging can also be used to assess for the presence of epicardial CAD in patients with cancer and in cancer survivors. In a study designed to establish the diagnostic accuracy of stress CMR in suspected CAD using invasive coronary angiography as the gold standard and to compare CMR to SPECT, the positive predictive value for stress CMR was 77.2% (95% confidence interval [CI], 72.1% to 81.6%) and the negative predictive value was 86.5% (95% CI, 81.8% to 90.1%). These positive and negative predictive values were similar to those obtained for SPECT, at 71.4% (95% CI, 65.3% to 76.9%) and 79.1% (95% CI, 74.8% to 82.8%), respectively. Modality availability and the ability to evaluate other clinical questions of interest can help ordering clinicians decide if CMR imaging or another modality, such as radionuclide MPI or stress echocardiography, would be most helpful for a particular patient.
Table 25.2 summarizes and compares the modalities for evaluation of epicardial CAD.