- •
Cardiac amyloidosis is often underdiagnosed, misdiagnosed, or diagnosed late in the natural course of the disease.
- •
Accurate diagnosis and typing of cardiac amyloidosis are both critical for initiation of appropriate therapy.
- •
Novel therapies appear to be more effective in early-stage ATTR cardiac amyloidosis and so early detection is important.
- •
Global longitudinal strain on echocardiography and expanded ECV on CMR are early markers of cardiac amyloid infiltration. Nevertheless, although characteristic features may accurately distinguish amyloid from nonamyloid heart disease, they are not adequate to distinguish AL from ATTR cardiac amyloidosis.
- •
Endomyocardial biopsy proof of amyloidosis and extracardiac biopsy proof with typical imaging features or with abnormal cardiac biomarkers (AL) can diagnose cardiac amyloidosis.
- •
A nonbiopsy diagnosis of cardiac ATTR amyloidosis is now possible and requires three components: (1) HF, (2) an echocardiogram or CMR with typical imaging features, and (3) the exclusion of AL amyloidosis by serum free light chain assay and serum and urine immunofixation electrophoresis studies.
- •
A nonbiopsy diagnosis makes possible screening for cardiac amyloidosis. Screening patients with clinical or imaging red flags for amyloidosis may help identify early disease.
- •
A diagnosis of ATTR cardiac amyloidosis based on 99m Tc-bone scintigraphy requires SPECT imaging to visualize myocardial tracer uptake grade 2 or 3 and to exclude blood pool activity. Imaging 2 to 3 hours after injection of the radiotracer minimizes interference from blood pool activity.
- •
Serum cardiac biomarkers, the eGFR, serum free light chain levels, and structural features on echocardiography, as well as increased semiquantitative myocardial uptake of 99m Tc-bone–avid SPECT tracers, are high-risk features.
- •
18 F-labeled amyloid tracer PET scans are positive in both AL and ATTR cardiac amyloidosis and offer the potential for quantitation of amyloid burden.
Introduction
The name amyloid is derived from the Latin word amylum (starch). Amyloidosis is a protein folding disorder that is characterized by extracellular deposition of a fibrillar protein in soft tissues, visceral organs, and the peripheral nervous system. Amyloid deposits progressively interfere with the structure and function of affected organs throughout the body. Amyloidosis is a life-threatening, progressive, infiltrative multisystem disease that is often underdiagnosed or misdiagnosed. There are two dozen proteins that are known to form amyloid fibrils in vivo. There are two predominant types that involve the heart: amyloid light chain (AL) amyloidosis, which is typically associated with plasma cell dyscrasia, and amyloid transthyretin (ATTR) amyloidosis. ATTR amyloidosis is further subdivided into variant (ATTRv) and wild-type (ATTRwt) forms.
AL amyloidosis is caused by abnormal immunoglobin light chains, which change into amyloid fibrils and undergo extracellular systemic deposition. Among the three types (AL, ATTRv, and ATTRwt), patients with cardiac AL amyloidosis have the worst prognosis. The median survival of untreated AL patients with heart failure (HF) is 6 months. ATTRv amyloidosis is caused by variants of the transthyretin (TTR) gene, which show an autosomal dominant inheritance pattern. The prognosis of cardiac ATTRv amyloidosis is better than that of AL amyloidosis. ATTRwt amyloidosis is caused by the accumulation of wild-type TTR. The median survival of ATTRwt is better than that of ATTRv patients. Effective disease-modifying therapies are now available to treat ATTRv and ATTRwt. Therefore, early detection is important to improve outcomes.
Epidemiology
AL amyloidosis is a rare disease. There are around 2500 new cases diagnosed each year. Of those with systemic AL amyloidosis, around 50% have cardiac involvement. ATTRv is not quite as rare. In fact, V122I is the most common TTR gene mutation in the United States. It is inherited as an autosomal dominant pattern and is predominantly present in African Americans. Approximately 3% to 4% of African Americans are carriers of this mutation, but penetrance is age dependent. There are approximately 25,000 to 120,000 cases in the United States. , ATTRwt is not rare. It is the most common of the three types and is classically described in older white males. ATTRwt is demonstrated in the hearts of approximately 25% of patients older than 80 years. Around 10% to 15% of elderly HF preserved ejection fraction (HFpEF) patients have ATTRwt, and it is estimated that there may be around 1 million patients with ATTRwt. Although there has been an increase in the detection of cardiac amyloidosis in the United States since 2001, data derived from death certificates and from Medicare fee-for-service data indicate substantial regional differences with underdiagnosis in the southern states.
Meanwhile, the nature of cardiac amyloidosis is changing. In the late 1990s to early 2000s, AL was the most common form, but now ATTR-cardiomyopathy (CM) is the most common. ATTRwt is the most prevalent form of ATTR-CM.
Clinical presentation
Clinical features
Cardiac amyloidosis commonly occurs in systemic amyloidosis patients. Patients have heterogeneous symptoms because of multiple-system involvement. The diagnosis may be expedited by recognizing clinical features such as:
- •
HFpEF without hypertension, particularly in men;
- •
evidence of right-sided HF,
- •
intolerance of angiotensin-converting enzyme (ACE) inhibitors or beta blockers,
- •
periorbital purpura or nephrotic range protein (both signs of AL),
- •
conduction disease in patients older than 50 years;
- •
atrial fibrillation/flutter,
- •
autonomic or sensory polyneuropathy,
- •
atypical multiple myeloma,
- •
bilateral carpal tunnel syndrome (in ATTR),
- •
lumbar spinal stenosis and biceps tendon rupture.
Biochemical features
No plasma or urine biomarker is available for the diagnosis of ATTR. Nevertheless, very high levels of N-terminal pro-brain natriuretic peptide (NT-proBNP) that are disproportionate compared with the degree of HF and elevated troponin levels in a patient with a hypertrophic phenotype on echocardiography are suggestive of TTR-CM. NT-proBNP is elevated early in ATTRv CM before cardiac symptoms appear, especially among asymptomatic carriers of ATTR gene mutations or patients with neurologic symptoms.
Electrocardiographic and imaging features
Electrocardiographic features
Electrocardiographic (ECG) features include an infarct pattern in the absence of coronary artery disease (CAD), low voltage, conduction disease, and atrial fibrillation.
Echocardiographic features
Echocardiographic features may include increased biventricular wall thickness, a thickened interatrial septum, valvular thickening, biatrial enlargement, diastolic dysfunction, restrictive phenotype, pericardial effusion, preserved ejection fraction (early), or systolic dysfunction (late). There may be apical sparing on the strain imaging (in the form of a bull’s eye or cherry on top) and a low myocardial contraction fraction. There is also a low voltage-to-mass ratio.
Magnetic resonance imaging features
In addition to the cardiac structural changes noted on echocardiography, magnetic resonance imaging (MRI) can provide specific clues to myocardial tissue characterization in amyloidosis. It may show diffuse late gadolinium enhancement (LGE), increased extracellular volume (ECV) and T1, and an inability to suppress the myocardial signal. LGE is seen in almost all patients with cardiac amyloidosis. The pattern of LGE can be transmural or subendocardial. , Atrial LGE and right ventricular (RV) LGE have also been described. Suboptimal myocardial nulling and altered gadolinium kinetics with myocardial nulling before blood pool nulling are characteristic features of amyloidosis. Increased ECV is also common in LGE and is an early marker of myocardial infiltration, which can be noted in myocardial segments without LGE. Native T1 times, a measure of myocardial tissue characteristics without the need for gadolinium, are increased in patients with cardiac amyloidosis AL and ATTR .
Radionuclide imaging features
Radionuclide imaging features will be in discussed in detail in the following sections.
Radionuclide imaging
The list of single photon emission computed tomography (SPECT) and positron emission tomography (PET) radiotracers for cardiac amyloidosis imaging is shown in Table 24.1 .
| Tracer Type | Tracer Name | SPECT or PET |
|---|---|---|
| Bone-seeking tracers | ||
| 99m Tc-pyrophosphate (PYP) | SPECT | |
| 99m Tc-hydroxymethylene diphosphonate (HMDP) | SPECT | |
| 99m Tc-3.3-diphosphono-1,2-propanodicarboxylic acid (DPD) | SPECT | |
| 18 F-sodium fluoride | PET | |
| Sympathetic innervation tracers | ||
| 123 I-metaiodobenzyl guanidine (mIBG) | SPECT | |
| Amyloid PET tracers | ||
| 11 C-Pittsburgh B compound | PET | |
| 18 F-florbetapir | PET | |
| 18 F-florbetapir | PET | |
| 18 F-florbetaben | PET |
Bone-seeking radiotracers
The underlying molecular mechanism for the uptake of 99m Technetium (Tc)-pyrophosphate (PYP), 99m Tc-3,3-diphosphono-1,2-propanodicarboxylic acid (DPD), or 99m Tc-hydroxymethylene diphosphonate (HMDP) in ATTR-CM has not been established. One hypothesis proposes that uptake of 99m Tc-labeled bone agents in ATTR-CM is due to binding of phosphate domains in these radiotracers to calcium in TTR amyloid fibrils. ,
There has been a long-standing interest in the use of 99m Tc-labeled bone-seeking radiotracers to diagnose cardiac amyloidosis. Some of the initial case reports and single-center cohort studies demonstrated variable utility ; however, this was before the use of molecular diagnostics to adequately distinguish AL and ATTR, as well as before the use of endomyocardial biopsy (EMB), mass spectroscopy, and serum and urine assays for monoclonal proteins and serum free light chain. More recent single-center studies have demonstrated that 99m Tc bone agents (both 99m Tc-DPD and PYP) are avidly taken by the hearts of patients with ATTR-CM and reported nearly 100% sensitivity and specificity. Moreover, a recent large international multicenter registry demonstrated that myocardial radiotracer uptake on bone scintigraphy was greater than 99% sensitive and 86% specific for ATTR-CM. They also showed that the combined findings of intense myocardial radiotracer uptake (grades 2 or 3) and the absence of a monoclonal protein in serum or urine had a specificity and positive predictive value for ATTR-CM of 100%. Finally, a recent multicenter registry showed that the presence of intense myocardial radiotracer uptake reflecting ATTR-CM may also have prognostic value. ,
99m Technetium imaging protocols
Several imaging protocols have been used for 99m Tc-labeled bone tracer cardiac scintigraphy studies. The most recent expert consensus document has provided recommendations for standardized imaging of 99m Tc-labeled bone tracer cardiac scintigraphy:
- •
The injected dose of 99m Tc-labeled bone tracers should be 10 to 25 mCi, depending on patient weight.
- •
The recommended delay between injection and scan is around 3 hours to allow for the blood pool radioactivity to clear. A 1-hour scan interval protocol with 99m Tc-PYP is optional with the understanding that a 3-hour delayed scan may be necessary in patients with high blood pool radioactivity at 1 hour.
- •
Planar imaging of the chest (anterior and lateral views using a 90-degree detector configuration) followed by SPECT imaging (360-degree or 180-degree) in the presence of any myocardial uptake is recommended, as is SPECT imaging alone. SPECT imaging is preferred because it helps overcome the overlap of bone radiotracer uptake over the heart, which improves quantification of the degree of myocardial uptake.
- •
Hybrid SPECT/computed tomography (CT) imaging, using a noncontrast low-dose CT for attenuation correction, may be superior to SPECT alone because it helps delineate blood pool radioactivity from myocardial uptake and provide attenuation-corrected images.
99m Tc image interpretation
- •
Image quality is assessed by examining the degree of radioactivity uptake in bone (sternum, ribs) and residual blood pool activity on SPECT images.
- •
The study is uninterpretable if there is no bone uptake, which can sometimes be seen in patients with poor renal function or with images obtained very early after radiotracer injection (<30 minutes).
- •
If excess blood pool activity is seen, particularly if images were acquired early at 1 hour postinjection, a repeat of the imaging at 3 hours is recommended.
- •
The pattern of myocardial 99m Tc uptake is reported as none, focal, diffuse, or focal on diffuse. Currently, diffuse or focal on diffuse uptake is considered positive.
- •
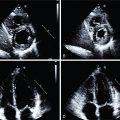
Semiquantitative interpretation in relation to rib uptake is recommended. Myocardial uptake of 99m Tc is graded as follows ( Fig. 24.1 ):
- •
Grade 0: absent myocardial uptake
- •
Grade 1: less than rib uptake
- •
Grade 2: equal to rib uptake
- •
Grade 3: greater than rib uptake

Fig. 24.1
99m Technetium (Tc) myocardial uptake semiquantitative visual scoring system.
99m Tc-PYP anterior planar images of the chest and selected axial and coronal SPECT and SPECT/CT images. Images demonstrate examples of visual grades from 0 to 3. A visual grade of 2 or greater is suggestive of ATTR cardiomyopathy. ATTR , Amyloid transthyretin; CT , computed tomography; PYP , pyrophosphate; SPECT , single photon emission computer tomography.
- •
Heart to contralateral ratio
A heart to contralateral (H/CL) ratio is derived by drawing a region of interest (ROI) over the left ventricle (LV) and another similar-sized ROI over the contralateral chest ( Fig. 24.2 ). The ROIs must be drawn above the diaphragm and away from the sternum. H/CL quantification is simpler on SPECT images because there is no overlap between bone and soft tissue. The LV ROI should include myocardium and blood pool. The RV should be excluded from the contralateral ROI. Mean counts in ROI over the LV divided by mean counts in an identical-size ROI over the contralateral chest provides the H/CL ratio. H/CL is used in patients with evident myocardial uptake of 99m Tc on SPECT images. An H/CL equal to or greater than 1.5 at 1 hour or equal to or greater than 1.3 at 3 hours is abnormal and considered diagnostic of ATTR-CM, provided systemic AL amyloidosis is excluded by serum free light chain assay and serum and urine immunofixation studies.

Sources of false-positive 99m Tc scans
The most common source of false-positive 99m Tc scans is the presence of increased blood pool radioactivity. As previously mentioned, the use of hybrid SPECT/CT helps overcome this problem (see Fig. 24.1 ). Other less frequent sources of false-positive scans include acute/subacute myocardial infarction, which may demonstrate focal uptake on SPECT or a low-grade positive scan on planar imaging; left-sided rib fracture or metastatic rib lesions with focal uptake on planar images, which can all be overcome with SPECT or SPECT/CT imaging; an apolipoprotein A1 mutation; and hydroxychloroquine toxicity with diffuse myocardial uptake.
Sources of false-negative 99m Tc scans
Early stages of ATTR-CM with low amyloid burden can potentially present without significant 99m Tc-PYP/DPD/HMDP uptake. Also, certain types of amyloid fibrils (full-length fibrils, particularly in patients with the mutations Phe64Leu and Thr59Lys) may also manifest as false-negative 99m 99mTc-PYP/DPD/HMDP scans. Similar to other radionuclide imaging applications, soft-tissue attenuation (e.g., breast implants), pericardial effusions, or pleural effusions can potentially limit the radioactivity signal from the heart, especially when using planar imaging.
Myocardial neuronal imaging radiotracers
123 I-meta iodobenzylguanidine (MIBG) is a norepinephrine analog that has been validated to study myocardial sympathetic denervation (please refer to detailed discussion in Chapter 21 ). In certain forms of hereditary ATTR cardiac amyloidosis that are characterized by neuropathic manifestations, MIBG imaging has been used to detect early cardiac denervation. A low heart-to-mediastinum ratio (<1.6) portends poor survival in patients with hereditary ATTR cardiac amyloidosis. Its prognostic value in AL and ATTRwt cardiac amyloidosis is not known.
Targeted amyloid imaging radiotracers
Several PET radiotracers originally developed for Alzheimer’s disease have been studied for imaging cardiac AL and ATTR amyloidosis (see Table 24.1 and Case Vignette 6). None of these tracers are approved for clinical imaging of cardiac amyloidosis. Although their exact binding site is not well known, the ability of these tracers to image amyloid formed from various precursor proteins, including AL, ATTR, and A-beta, suggests that it may bind to the beta-pleated structure of the amyloid fibril.
Imaging protocols
Typical imaging protocols for 11 C Pittsburgh Compound-B (PiB) and 18 F-amyloid tracers include a list mode image acquisition with reconstruction of dynamic and static images. A process of dedicated cardiac bed position image acquisition followed by partial whole-body images is typical and helps to identify cardiac and extracardiac amyloid infiltrates. Cardiac images are reconstructed and oriented into standard cardiac imaging planes of short-axis, vertical, and horizontal long-axis images. Most of the literature has focused on the 10- to 30-minute images to calculate retention index and to evaluate the static images.
Image interpretation
Radiotracer uptake is interpreted visually by comparing a signal in the myocardium to the activity in the blood pool. Quantitation is performed using the standardized uptake values (SUVs), target-to-background ratio, and myocardial retention index.
The literature findings suggest that amyloid PET tracers are excellent for imaging cardiac amyloidosis and for distinguishing control patients from those with amyloidosis. Indeed, one study using both 99m Tc-DPD and 11 C-PiB imaging in patients with hereditary cardiac amyloidosis found 11 C-PiB in the myocardium in patients without DPD uptake. This supports their ability to detect amyloid fibrils, as opposed to 99m Tc-DPD, which is known to better image fragmented fibrils rather than full-length fibrils (which are more common in certain ATTRv patients). Although most studies also suggest that the amyloid PET tracer avidity is greater with AL than with ATTR, these two entities cannot be distinguished using the amyloid PET tracers. Pilot data have suggested that the intensity of the tracer in the myocardium may be lower in treated AL patients compared with untreated AL patients. One pilot study suggests that myocardial 18 F-florbetapir uptake may predict outcomes in patients with AL cardiac amyloidosis. Although not an amyloid-targeted agent, a limited literature with 18 F-NaF (a bone-seeking radiotracer) and PET suggests low target-to-background ratios (<1.0) for imaging ATTR and AL cardiac amyloidosis.
Diagnostic criteria and appropriate use of imaging
Until recently, amyloidosis was an underdiagnosed disease, and most of the studies had been performed in highly selected cohorts of patients referred for evaluation to sites with expertise in cardiac amyloidosis using site-specific protocols. This resulted in several site-specific protocols and no standardized imaging approaches. Despite the emerging large body of literature supporting the role of imaging in the evaluation and management of cardiac amyloidosis, diagnostic criteria for ATTR cardiac amyloidosis that incorporate advanced imaging features had not been defined. For similar reasons, beyond the expert centers, there was a lack of knowledge on appropriate clinical use of imaging in cardiac amyloidosis. Therefore the American Society of Nuclear Cardiology in collaboration with multiple other societies developed a document addressing standardized imaging protocols, diagnostic criteria, and appropriate use of imaging in cardiac amyloidosis. ,
Standardized imaging protocols
The consensus recommendations have developed standardized protocols for 99m Tc-PYP, 99m Tc-DPD, and 99m Tc-HMDP.
Diagnostic criteria for cardiac amyloidosis
Cardiac amyloidosis can be diagnosed using:
- 1.
Endomyocardial biopsy with typing of the amyloid fibril using immunohistochemistry or more accurately using mass spectrometry–based methods.
- 2.
Extracardiac biopsy with:
- a.
Typical cardiac imaging features (for ATTR cardiac amyloidosis) or
- b.
Typical cardiac imaging features or elevated cardiac biomarkers of NT proBNP or troponin T (for AL cardiac amyloidosis) and other causes for cardiac biomarker release are excluded.
- a.
- 3.
A nonbiopsy diagnosis is feasible for ATTR cardiac amyloidosis in patients that meet four criteria:
- a.
HF,
- b.
Typical echocardiogram or cardiac magnetic resonance (CMR) imaging features,
- c.
Grade 2 or 3 uptake of 99m Tc-PYP, 99m Tc-DPD, or 99m Tc-HMDP, and
- d.
Systemic AL amyloidosis has been excluded by serum free light chain assay and serum and urine immunofixation electrophoresis.
- a.
Appropriate use criteria for imaging cardiac amyloidosis
Seven amyloidosis experts rated the appropriateness of several indications for imaging using a modified Delphi approach. Based on those ratings, indications were categorized as appropriate, maybe appropriate, or rarely appropriate. CMR was rated appropriate for most scenarios where new-onset HF was present. Radionuclide imaging with 99m Tc bone tracers ( 99m Tc-PYP, 99m Tc-DPD, and 99m Tc-HMDP) was rated as rarely appropriate for clinical scenarios consistent with AL amyloidosis and appropriate in clinical scenarios suggestive of ATTR cardiac amyloidosis, including:
- •
Age greater than 60 years,
- •
African Americans with HFpEF or LV thickening
- •
Non–African Americans with HFpEF and LV thickening
- •
- •
Low-flow low-gradient aortic stenosis
- •
HF with unexplained peripheral sensory neuropathy
- •
Known or suspected hereditary ATTR cardiac amyloidosis
- •
Monoclonal gammopathy or elevated free light chain levels
- •
Follow-up for progressive symptoms in known AL or ATTR
- •
Other clinical scenarios rated appropriate for echocardiogram evaluation:
- •
Carpal tunnel syndrome
- •
Biceps tendon rupture
- •
Lumbar spinal stenosis
- •
Patient-centered applications of imaging in cardiac amyloidosis
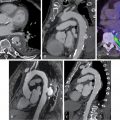
Case vignette 1: A male patient with new-onset heart failure
A 74-year-old male with hypertension on hydrochlorothiazide 25 mg orally daily and history of two prior surgeries for carpal tunnel syndrome (10 and 8 years prior) presented for evaluation of HF before a scheduled left total knee replacement. His NT-proBNP was 3900 pg/mL (normal limit: <900 pg/mL) and troponin T was 0.06 ng/mL (normal limit: <0.01 nl/mL). ECG, transthoracic echocardiography (TTE), and CMR results ( Figs. 24.3 A–C) demonstrated classic features of cardiac amyloidosis. The echocardiogram showed LV thickening (14-mm walls) with an LVEF of 60%. A CMR with gadolinium contrast confirmed thick LV (16 mm) and showed diffuse LGE and substantially expanded ECV (>0.041 in each of the 17 myocardial segments). A fat pad biopsy was negative. Because of high clinical suspicion of ATTR cardiac amyloidosis, he was referred for a 99m Tc-PYP scan, which was strongly positive (see Fig. 24.3 D). Immunofixation electrophoresis studies of the serum and urine and serum free light chain assay were negative, excluding AL amyloidosis. Genetic testing showed no TTR mutations. A final diagnosis of ATTRwt cardiac amyloidosis was made.