Pediatric Abdomen and Pelvis
Susan D. John
Leonard E. Swischuk
Gastrointestinal Tract
Gastrointestinal Obstruction
Gastrointestinal obstruction is a relatively common problem in infants and children and must be distinguished from numerous other causes of vomiting and abdominal distention. The causes of intestinal obstruction in children are widely varied in urgency and management implications, and imaging plays a critical role in prompt treatment of such conditions. The most likely causes of obstruction in children shift in importance according to age, and therefore, it is helpful to consider the various causes of obstruction within four age-group categories (Table 51.1). Determination of the level of obstruction also helps to infer the possible etiology. Abdominal radiographs continue to be a useful initial screening examination for assessing the site of obstruction and determining the need for further imaging.
Hypopharyngeal/upper esophageal obstruction is uncommon in infants and children but may be caused by a spasm of the cricopharyngeus muscle. Cricopharyngeal spasm may be related to neurologic dysfunction (e.g., Chiari malformation, cerebral palsy) or inflammation resulting from gastroesophageal reflux. Lack of normal cricopharyngeus muscle relaxation disturbs the well-coordinated swallowing mechanism and can lead to aspiration. In refractory cases, surgical division of the muscle may be required.
Difficulties with swallowing also can occur with inflammatory processes such as epiglottitis, retropharyngeal abscess, tonsillar abscess, or a number of tumors or cysts that occur in this area. A large pharyngeal diverticulum may produce obstruction. These diverticula can be congenital or iatrogenic due to perforation of the hypopharynx during intubation. They are best demonstrated with barium swallow.
Esophageal Obstructions.
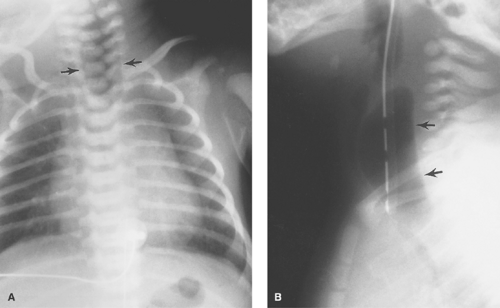
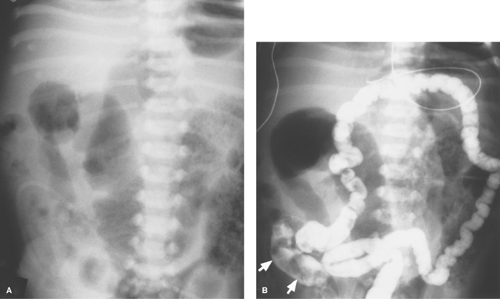
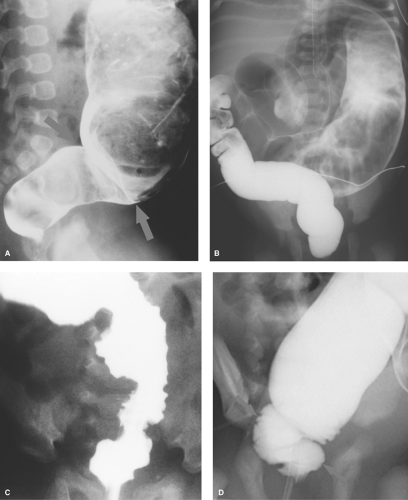
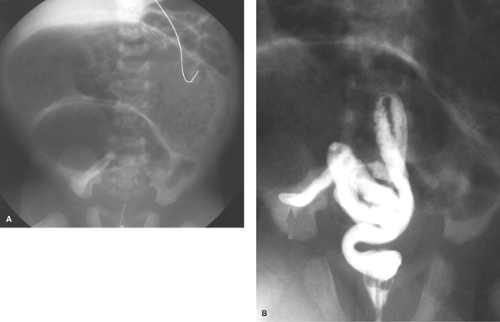
Esophageal Atresia and Tracheoesophageal Fistula. The most common congenital obstruction of the esophagus is esophageal atresia (Table 51.2). This anomaly is a result of faulty development and separation of the embryonic foregut early in gestation. The site of atresia is usually in the upper third of the esophagus. The air-filled upper esophageal pouch is often visible on radiographs (Fig. 51.1). The distended proximal esophageal pouch may cause pressure on the trachea during fetal development, resulting in focal tracheomalacia.
Esophageal atresia is frequently associated with tracheoesophageal fistula. Most commonly, the fistula extends obliquely from the trachea, just above the carina, to the distal esophageal pouch. The fistula allows air to enter the stomach and intestines, in some cases in large volumes. Air in the GI tract differentiates this type of esophageal atresia from isolated esophageal atresia without tracheoesophageal fistula, in which the stomach and intestines remain gasless. The tracheoesophageal fistula may also develop without esophageal atresia. Such fistulas should be carefully sought on esophagrams performed on infants with choking or respiratory difficulty during feeding (Fig. 51.2). Much less common, esophageal atresia may be accompanied by a fistula from the proximal esophageal pouch to the trachea. A small amount of barium may be placed in the proximal esophageal pouch with an end-hole catheter, in order to demonstrate such fistulas.
Esophageal atresia is more common in infants with trisomy 21 and may be associated with vertebral anomalies, duodenal atresia, anao rectal malformations, and other features of the VACTERL association. The prognosis of infants with esophageal atresia depends primarily on the severity of associated anomalies and on the length of the atretic segment. Surgical repair is more challenging with long-gap atresia. Common complications of esophageal atresia repair include anastomotic strictures (40%), anastomotic leakage (14% to 21%), and recurrent fistula (3% to 14%). Esophageal peristalsis is abnormal in patients with esophageal atresia, and GER is very common.
A variety of communications may exist between the esophageal pouch and the spine, ranging from fibrous bands to actual fistulae. If the communication involutes at both ends and only the central portion remains, the result is a neurenteric cyst. In other cases, an esophageal communication persists and a diverticulum is formed that extends into the spine or spinal canal. Faulty separation of the trachea and esophagus may also result in fistulae, fibrous bands, or diverticula. Tracheoesophageal fistula located high in the esophagus without esophageal atresia is the third most common abnormality in this group of lesions, following esophageal atresia with a distal
tracheoesophageal, and isolated esophageal atresia. The fistula is usually identified with barium swallow extending anteriorly from the esophagus to the lower trachea (Fig. 51.2).
tracheoesophageal, and isolated esophageal atresia. The fistula is usually identified with barium swallow extending anteriorly from the esophagus to the lower trachea (Fig. 51.2).
Congenital esophageal stenosis is a far less common cause of congenital esophageal obstruction. As in esophageal atresia, esophageal stenosis arises from faulty tracheal and esophageal separation where tracheobronchial cartilage remnants remain in the wall of the esophagus. On esophagram, a circumferential segment of narrowing with tapered margins is present. In older children and adolescents, the narrowed segment often contains characteristic concentric ring-like indentations.
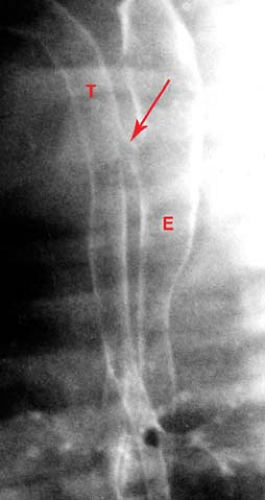 Figure 51.2. Tracheoesophageal Fistula. The trachea (T) and esophagus (E) are connected by a fistula (arrow). |
Congenitally short esophagus with intrathoracic stomach is not truly a congenital lesion. Even though seen at birth, this condition more likely represents the aftermath of chronic hiatal hernia during fetal life with GER and subsequent esophageal stricture leading to shortening (Fig. 51.3). Other uncommon congenital causes of esophageal obstruction include esophageal webs and diverticula. Esophageal obstruction can also result from compression of the esophagus by extrinsic masses or anomalous vessels.
Table 51.1 Most Common Causes of Gi Tract Obstruction By Age | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
Acquired esophageal obstructive lesions are primarily strictures or foreign bodies. Chronic foreign bodies are often nonradiopaque and can result in a stricture with pseudodiverticulum
(Fig. 51.4A). Esophageal neoplasms are extremely rare in infants and children and malignant neoplasms are virtually nonexistent.
(Fig. 51.4A). Esophageal neoplasms are extremely rare in infants and children and malignant neoplasms are virtually nonexistent.
Table 51.2 Causes of Esophageal Obstruction | ||||||
|---|---|---|---|---|---|---|
|
Peptic esophagitis is associated with gastroesophageal reflux (GER) and can be seen with or without hiatus hernia. Although GER is very common in infants, peptic esophagitis with stricture is an uncommon complication. GER may be primary (chalasia), due to a lax gastroesophageal sphincter, or secondary to a gastric outlet obstruction. Causes of gastric obstruction (pylorospasm, pyloric stenosis, gastric diaphragm, gastric ulcer disease) must be excluded in infants with severe GER. GER is most reliably identified with 24-hour esophageal pH and impedance monitoring, but this procedure can be cumbersome and gives no direct information about obstruction. Nuclear gastric reflux studies are also sensitive. US with color Doppler can be used to detect GER, but the technique has not gained widespread popularity (1). Contrast upper GI examinations are useful to evaluate patients with duodenal obstruction but are generally not necessary to document GER in an infant with simple reflux, unless surgery is being planned.
Peptic esophageal strictures usually are short and located in the distal third of the esophagus. The occasional case of Barrett esophagus with a high stricture can also be encountered. Peptic strictures may be irregular or surprisingly smooth mimicking the findings of achalasia (Fig. 51.4B). Achalasia is uncommon as a cause of distal esophageal obstruction in children.
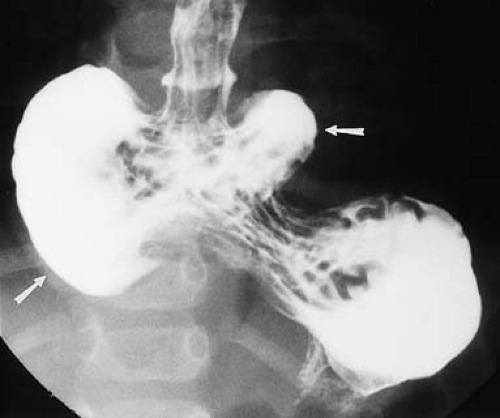 Figure 51.3. Intrathoracic Stomach. A large portion of the stomach lies above the diaphragm (arrows). Note reflux into a dilated, shortened esophagus with thickened mucosa suggestive of inflammation. |
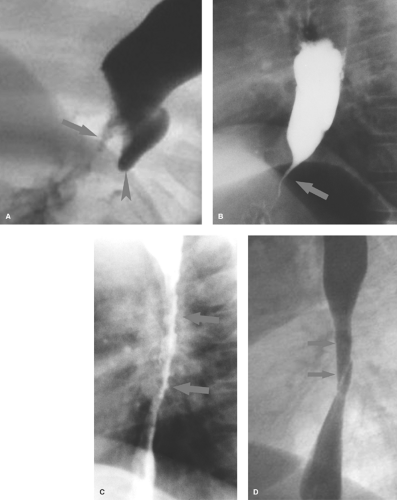
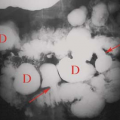
Caustic esophagitis with stricture usually results from accidental ingestion of alkaline substances such as sodium hydroxide, potassium hydroxide (lye), or alkaline disk batteries. Disk batteries can become lodged in the esophagus and leak their alkaline contents, producing deep burns of the mucosa and submucosa. All alkaline burns cause deep penetrating injury that commonly results in stricture. Acids, even swallowed in significant quantities, produce more superficial burns. While mucosal injury may be extensive, deep mural injury with fibrotic stricture is less common. Lye strictures lead to long areas of irregular narrowing (Fig. 51.4C). Esophageal burns caused by an ingested battery or medication (aspirin, tetracycline, clinitest tablets) result in a more focal stricture.
Epidermolysis bullosa is a hereditary condition characterized by inflammatory skin and mucosal lesions that can heal with fibrosis resulting in esophageal stricture or web (2) (Fig. 51.4D).
Acute Esophagitis. Other forms of esophageal inflammation are uncommon in children. Acute inflammation with spasm occurs with infectious esophagitis, caused by organisms such as candida or herpes. These types of esophagitis are more common in immunocompromised children. Eosinophilic esophagitis is thought to be allergic in nature and is most commonly seen in children with asthma.
Gastric Obstruction.
Congenital obstructing lesions of the stomach are far less common than congenital obstructing lesions elsewhere in the GI tract (Table 51.3). Gastric distention on radiographs does not always indicate obstruction in infants. A large gas-filled stomach is commonly seen in normal infants, and persistent asymptomatic gastric distention occurs in infants receiving prostaglandins for ductal-dependent congenital heart disease (3).
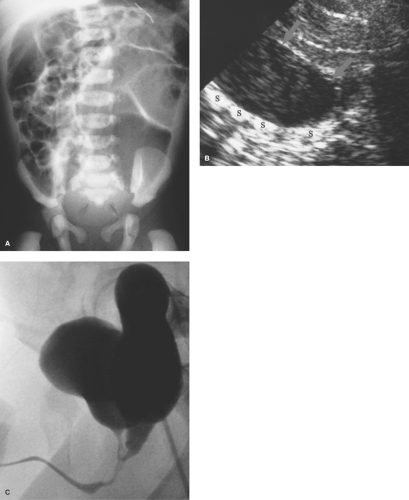
Gastric atresia is believed to result from a vascular insult to the stomach in utero. In the newborn infant, if obstruction of the stomach is complete, radiographs show no air distal to the stomach (Fig. 51.5). Gastric atresia usually occurs at the level of the pylorus. In some cases, atresia takes the form of a gastric diaphragm or membrane which, if incomplete, will allow some gas distal to the obstructing web. US or an upper GI series can be used to identify the incomplete diaphragm.
Gastric atresia may occur in infants with congenital epidermolysis bullosa because of inflammatory stricture. Microgastria occurs with other GI atresias or VATER syndrome and is commonly associated with the polysplenia/asplenia syndromes.
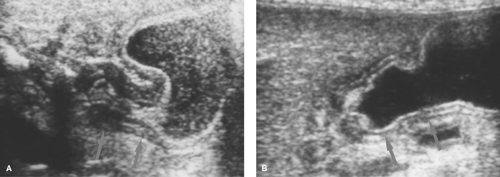
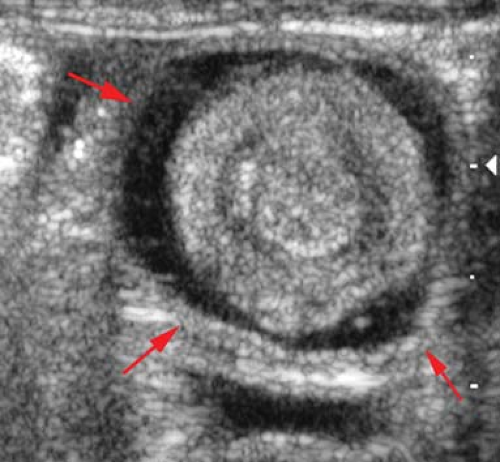
Gastric duplications must be critically located in the antrum or be very large to result in obstruction. They are best demonstrated with US where they appear sonolucent with a wall that demonstrates both mucosal and muscular layers (see Fig. 51.70).
Table 51.3 Causes of Gastric Obstruction | |||||||||
|---|---|---|---|---|---|---|---|---|---|
|
Gastric volvulus is an uncommon cause of gastric obstruction in children. The volvulus may be idiopathic or may be associated with congenital conditions that involve abnormal position of the stomach, such as diaphragmatic hernia, diaphragmatic eventration, or asplenia syndrome. Gastric volvulus may be classified as organoaxial, in which the stomach rotates along its longitudinal axis, or mesoaxial, in which it rotates about a line perpendicular to the cardiopyloric line. Radiographs may show marked gaseous distention and dilatation of the stomach (Fig. 51.6), which may extend into the chest if volvulus is associated with a diaphragmatic or paraesophageal hernia. Gastric volvulus should be considered an acute surgical emergency; however in some children volvulus may be chronic.
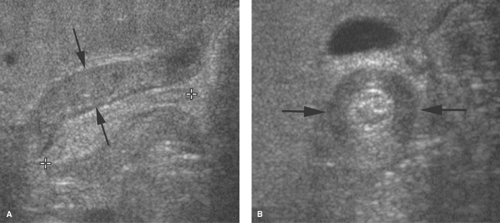
Pylorospasm is a reactive problem secondary to insult to the gastric mucosa or muscle contraction from other causes of stress. Mucosal inflammation may be caused by milk allergy or peptic disease with ulceration. Real-time US demonstrates persistent contraction of the antropyloric region and poor emptying of liquids from the stomach. Mild (<3 mm) thickening of the outer circular muscle occasionally can be seen (Fig. 51.7). Muscle thickening greater than 3 mm is only a transient phenomenon in pylorospasm (4). During sonography, the antropylorus relaxes causing the muscle thickness to return to
normal and allowing gastric contents to pass into the duodenum. The findings can be demonstrated with upper GI series, but US usually suffices.
normal and allowing gastric contents to pass into the duodenum. The findings can be demonstrated with upper GI series, but US usually suffices.
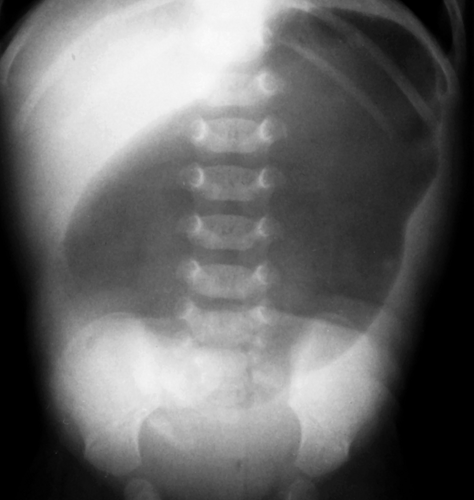 Figure 51.5. Gastric Atresia. Typical findings consist of a dilated air-filled stomach, with no air distal to the pylorus. |
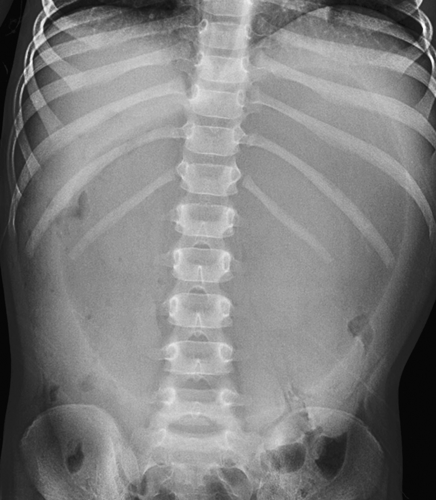 Figure 51.6. Gastric Volvulus. Massive enlargement of the stomach in this infant was caused by gastric volvulus. |
Hypertrophic pyloric stenosis is considered to be an acquired condition that may be related to prolonged pylorospasm. Failure of relaxation may be related to abnormal nerve cells or fibers, nitric oxide synthetase activity, or mRNA production (5). The condition most often develops between 2 and 10 weeks of age and is characterized by circumferential thickening of the pyloric muscle. US is the preferred examination because of its ability to directly assess the thickness of the pyloric muscle and to provide real-time evaluation of contraction of the pyloric canal (6). The hypertrophied pyloric muscle measures 3 mm or more in thickness while the pyloric canal is elongated beyond 14 mm (Fig. 51.8). The pylorus is in fixed spasm and very little fluid passes through it. In atypical cases, the pyloric canal is fixed in spasm, but the muscle measures 2 to 3 mm in thickness, whereas normal muscle measures no more than 1.5 mm in thickness. These patients may be treated medically for pylorospasm but should be followed closely, as some may progress to classic pyloric stenosis.
Tangential imaging of the normal pyloric canal or imaging with inadequate distention of the stomach with fluid may result in an erroneous impression of muscle thickening. Furthermore, it has been demonstrated in pyloric stenosis that at the 6 and 12 o’clock positions, the pyloric muscle may not be as hypoechoic as it is at the 3 and 9 o’clock positions. As
the circular muscle passes over the 6 and 12 o’clock positions, more acoustic interfaces are encountered and more echogenicity results. Finally, if the stomach is overfilled, the pyloric muscle mass can assume a posterior, upwardly curving position, making it more difficult to identify (7).
the circular muscle passes over the 6 and 12 o’clock positions, more acoustic interfaces are encountered and more echogenicity results. Finally, if the stomach is overfilled, the pyloric muscle mass can assume a posterior, upwardly curving position, making it more difficult to identify (7).
Gastric tumors are quite uncommon, but produce the same findings as in adults. Gastric teratomas can occur in the neonate and can be quite large at presentation.
Gastric bezoars are masses or retained gastric solid contents that may result in gastric outlet obstruction. Bezoars may consist of hair (trichobezoar), milk products (lactobezoar), vegetable material (phytobezoar), or cloth that is chronically chewed and swallowed, especially by developmentally delayed children. Air or barium outlining the bezoar is diagnostic (Fig. 51.9A). On US, an echogenic arc over the bezoar is characteristic (8). The arc is caused by echoes from the layer of air trapped between the bezoar and the gastric wall. CT shows an air-containing mass that is not attached to the gastric wall (Fig. 51.9B). Bezoars can sometimes extend into the duodenum and small bowel (9,10).
Duodenal Obstruction.
Congenital duodenal obstructions are more common than acquired obstructions in pediatric patients (Table 51.4). Radiographs localize the level of obstruction, which determines whether further imaging is required.
Table 51.4 Causes of Duodenal Obstruction | |||||||
|---|---|---|---|---|---|---|---|
|
Duodenal Atresia/Annular Pancreas. In a normal infant, gas passes from the stomach into small bowel during the first hours of life. When the stomach and duodenal bulb are distended with gas (“double bubble” sign) and no gas is present
distally (Fig. 51.10), the best diagnostic possibilities are duodenal atresia or annular pancreas. No further imaging is required. Rarely, a small amount of air may be seen in the distal GI tract in duodenal atresia with an anomalous Y configuration of the hepatopancreatic duct. The upper limb connects to the pre-atretic duodenum, while the lower limb connects to the post-atretic duodenum allowing air to pass into the distal small bowel. Air distal to a double-bubble obstructive pattern also can be seen with duodenal stenosis or duodenal web with a central perforation (Fig. 51.11). Contrast studies are indicated in these cases to distinguish them from midgut volvulus. US can demonstrate the dilated duodenal bulb with any cause of duodenal obstruction when the duodenum is filled with fluid.
distally (Fig. 51.10), the best diagnostic possibilities are duodenal atresia or annular pancreas. No further imaging is required. Rarely, a small amount of air may be seen in the distal GI tract in duodenal atresia with an anomalous Y configuration of the hepatopancreatic duct. The upper limb connects to the pre-atretic duodenum, while the lower limb connects to the post-atretic duodenum allowing air to pass into the distal small bowel. Air distal to a double-bubble obstructive pattern also can be seen with duodenal stenosis or duodenal web with a central perforation (Fig. 51.11). Contrast studies are indicated in these cases to distinguish them from midgut volvulus. US can demonstrate the dilated duodenal bulb with any cause of duodenal obstruction when the duodenum is filled with fluid.
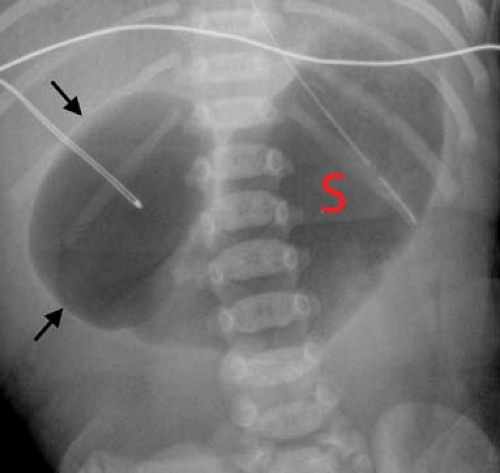 Figure 51.10. Duodenal Atresia. The classic double-bubble sign consists of a distended duodenal bulb (arrows) and a distended stomach (S). |
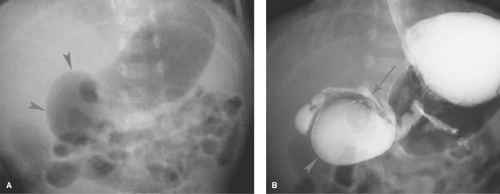
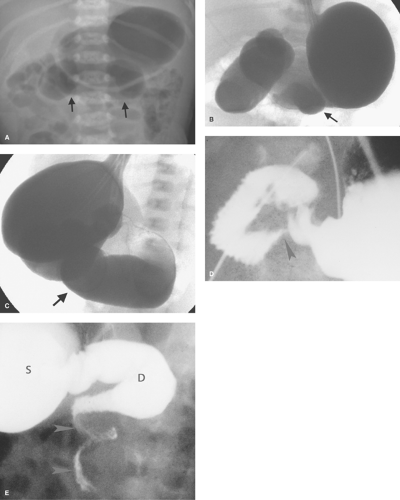
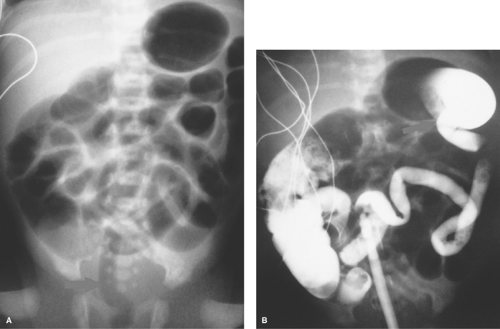
Midgut Volvulus. When the obstruction of the duodenum is located at the third or fourth portions of the duodenum, the most likely causes are duodenal diaphragm or intestinal malrotation with midgut volvulus or an obstructing peritoneal band. Midgut volvulus is a complication of intestinal rotational anomalies that are accompanied by abnormal position and poor fixation of the duodenum and small bowel. Rotational abnormalities of the intestines are developmental abnormalities that may vary from isolated poor fixation of the duodenum to complete nonrotation of the intestine with the colon residing in the left abdomen and small bowel on the right (11,12). A narrow peritoneal attachment in such patients may allow the small bowel to become twisted by active peristalsis. Volvulus is most likely to occur in infants with malrotation during the first year of life (13). The site of most proximal obstruction usually resides in the mid portion of the duodenum. The stomach and duodenum may appear dilated on radiographs, and gas is usually present in the distal bowel (Fig. 51.12A). In the supine position, the distended duodenum may contain fluid rather than air, erroneously suggesting a gastric obstruction. In other patients, the abdominal radiograph may appear normal. A contrast study is mandatory to determine whether volvulus is present. The upper GI series is most commonly used to directly demonstrate the obstruction of the duodenum, which characteristically occurs at the level of the third duodenal segment with midgut volvulus (Fig. 51.12B). Lateral views are helpful to demonstrate that the distal duodenal segments do not lie in a normal retroperitoneal location (Fig. 51.12C). A beak deformity may be seen at the point of obstruction in some cases (Fig. 51.12D), but in other patients the point of obstruction may show a smooth contour (see Fig. 51.12C,D). If obstruction of the duodenum is incomplete, spiraling of the twisted proximal small bowel may be seen (Fig. 51.12E).
US can be used to show the dilated duodenum and verify that the distal duodenum lies anterior to the superior mesenteric artery, indicating that it is intraperitoneal and malrotated (14). The superior mesenteric artery often lies in an atypical location anterior or to the left of the superior mesenteric artery (Fig. 51.13A), but this finding is not highly reliable. A spiral, “whirlpool” appearance vessels with Doppler US is also suggestive of volvulus (15,16). Contrast-enhanced CT shows the abnormal vascular position in addition to findings of bowel infarction when present (17). In the past, the barium enema
was commonly used to show the cecum displaced high into the mid abdomen, just under the transverse colon (Fig. 51.13B). However, because the cecum tends to be mobile in normal infants, any position lower than the transverse colon cannot be interpreted as representative of midgut volvulus.
was commonly used to show the cecum displaced high into the mid abdomen, just under the transverse colon (Fig. 51.13B). However, because the cecum tends to be mobile in normal infants, any position lower than the transverse colon cannot be interpreted as representative of midgut volvulus.
Total obstruction at the third portion of the duodenum also can be seen with peritoneal bands (Ladd bands) that frequently accompany rotational abnormality of the intestines. These bands may produce an oblique indentation of the third or fourth portion of the duodenum. Other congenital
abnormalities leading to obstruction at this level are duodenal webs and diaphragms, enteric duplication cyst, and, rarely, a preduodenal portal vein. Intestinal malrotation can be present without midgut volvulus, shown by an abnormal location of the duodenojejunal junction but no dilatation or obstruction to flow of contrast (Fig. 51.13C). Such cases are not surgical emergencies, but a Ladd procedure to surgically fix the small bowel within the peritoneum is usually performed electively. In such cases, it is prudent to follow the contrast material into the colon or perform a contrast enema to ascertain the position of the cecum, which infers the width of the mesentery (18,19).
abnormalities leading to obstruction at this level are duodenal webs and diaphragms, enteric duplication cyst, and, rarely, a preduodenal portal vein. Intestinal malrotation can be present without midgut volvulus, shown by an abnormal location of the duodenojejunal junction but no dilatation or obstruction to flow of contrast (Fig. 51.13C). Such cases are not surgical emergencies, but a Ladd procedure to surgically fix the small bowel within the peritoneum is usually performed electively. In such cases, it is prudent to follow the contrast material into the colon or perform a contrast enema to ascertain the position of the cecum, which infers the width of the mesentery (18,19).
Duodenal hematoma is perhaps the most common acquired cause of duodenal obstruction. Hematomas usually result from blunt abdominal trauma, commonly caused by motor vehicle collisions, bicycle handlebar injuries, or child abuse. CT or US can demonstrate the asymmetrical or concentric thickening of the duodenal wall at the site of the hematoma (Fig. 51.14A,B). Findings suggestive of duodenal laceration on CT include retroperitoneal air or fluid (Fig. 51.14C) (20), but retroperitoneal fluid can also be seen with duodenal injury without perforation (Fig. 51.14D). The findings may be confirmed by contrast duodenography (Fig. 51.14E). Intramural hematomas may also occur in children with hemophilia or Henoch–Schöenlein purpura or may be a complication of duodenoscopy with biopsy.
Obstruction of the duodenum secondary to peptic ulcer disease is rare in children. Duodenal tumors are also rare; however, extrinsic masses occur and may cause obstruction. The duodenum is a common site of origin for intestinal duplication cysts.
Small Intestinal Obstruction.
Small intestinal obstruction in the neonate and young infant is more likely to be congenital while, in the older child, acquired problems are more common (Table 51.5). In the neonate, it is difficult to distinguish distal small bowel obstruction from colonic obstruction on plain radiographs. Obstruction anywhere between the ileum and the rectum can produce a similar pattern of numerous dilated loops of intestine. A contrast enema should be performed to better evaluate the cause of obstruction.
Jejunal Atresia. Jejunal and ileal atresia are caused by the interruption of mesenteric blood supply during fetal development. Radiographs demonstrate a variable number of dilated loops of jejunum, depending on the level of atresia, with no gas distally (Fig. 51.15). Often no further contrast studies are required. A variation of intestinal atresia, described as apple peel small bowel, consists of diffuse atresia of the small bowel with multiple sites of severe stenosis and a spiral configuration of the atretic segment. This condition tends to be familial.
Segmental volvulus of the small bowel can occur anywhere along its length, but is an uncommon cause of jejunal obstruction. Similarly, internal hernias are rare.
Segmental volvulus of the small bowel can occur anywhere along its length, but is an uncommon cause of jejunal obstruction. Similarly, internal hernias are rare.
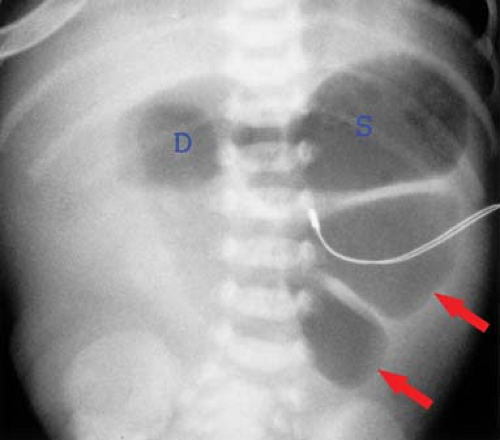 Figure 51.15. Jejunal Atresia. Conventional radiograph shows distention of the stomach (S), duodenum (D), and loops of the upper intestine (arrows). No air is present distal to the proximal jejunum. |
Ileal atresia and meconium ileus are the most common causes of distal small bowel obstruction in the neonate. In both conditions, contrast studies reveal a characteristic generalized microcolon, indicating that meconium has not passed normally into the colon during fetal life. Meconium ileus is usually the earliest manifestation of cystic fibrosis (21), resulting from abnormally thick masses of meconium that cannot pass through the ileocecal valve. Plain radiographs demonstrate multiple dilated loops of small intestine with bubbly intestinal contents representing retained meconium (Fig. 51.16A). When air-fluid levels are present in the dilated small bowel, ileal atresia is the more likely diagnosis. It is important to distinguish between the two conditions, for ileal atresia is corrected surgically while meconium ileus is often treated with water-soluble contrast enema. When performing such an enema, contrast material should reflux into the terminal ileum to outline, as well as lubricate, the inspissated meconium (Fig. 51.16B). The enema should be performed carefully because of the increased risk of perforation of a microcolon. If contrast does not reflux into the terminal ileum, surgery is required for definitive diagnosis and evacuation of the obstructing meconium.
Table 51.5 Causes of Small Intestinal Obstruction | |||||||
|---|---|---|---|---|---|---|---|
|
Meconium plug syndrome is a misleading name for a condition caused by functional immaturity and abnormal peristalsis of the distal colon. Also known as the small left colon syndrome, this condition should not be confused with meconium ileus. In the meconium plug syndrome, a normal to dilated proximal colon filled with meconium and an empty distal descending colonic segment are characteristic (Fig. 51.17). The meconium in infants with this condition is normal and is not the cause of obstruction. This condition is more common in normal large
infants and infants of diabetic mothers. The functional obstruction is transient and can often be treated by rectal stimulation or saline enemas. In more persistent cases, an enema using contrast that contains Tween 80 (such as dilute Gastrografin) can be used to irritate the colon and stimulate defecation. Normal ganglion cells are present in these infants, and once the meconium has passed, the patient will defecate normally.
infants and infants of diabetic mothers. The functional obstruction is transient and can often be treated by rectal stimulation or saline enemas. In more persistent cases, an enema using contrast that contains Tween 80 (such as dilute Gastrografin) can be used to irritate the colon and stimulate defecation. Normal ganglion cells are present in these infants, and once the meconium has passed, the patient will defecate normally.
Hirschsprung disease can resemble meconium plug syndrome radiologically, but the cause and prognosis is very different. In meconium plug syndrome, the obstruction usually resolves rapidly after the enema examination, whereas the obstruction generally persists in children with Hirschsprung disease.
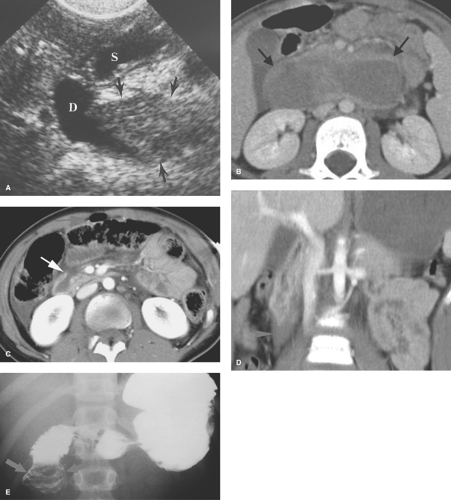
Incarcerated inguinal hernia is a common cause of low intestinal obstruction between 1 and 6 months of age. The findings of intestinal obstruction on radiographs are characteristic, and the key to radiologic diagnosis is visualization of a unilaterally prominent inguinal fold or loops of air-filled bowel in the scrotum (Fig. 51.18). US can identify the incarcerated intestine in the inguinal canal or scrotum and can differentiate a hernia from other causes of scrotal swelling.
Intussusception. After 6 months of age, intussusception becomes an increasingly important acquired cause of intestinal obstruction. In young children, most intussusceptions are ileocolic and the cause is idiopathic. Redundant, inflamed mucosa or lymph nodes may act as the lead point. Definable lead points such as diverticula, polyps, or tumors are more commonly encountered in neonates and in older children. Intussusception can occur postoperatively or as a complication of conditions that cause bowel wall thickening, such as Henoch–Schöenlein purpura or cystic fibrosis.
Abdominal radiographs may be normal or may demonstrate intestinal obstruction (22). In approximately half the cases, the head of the intussusceptum is visible on radiographs as soft tissue mass effect along the course of the colon (Fig. 51.19A). Fat trapped within the intussusceptum may be visible on radiographs. US is a very effective and reliable imaging modality for the demonstration of intussusception (23). Characteristically, a cylindrical mass is seen, consisting of an outer hypoechoic ring surrounding tissues of variable echogenicity. Concentric rings may be seen representing layers of edematous intestine alternating with layers of mesentery (24). Often anechoic fluid, echogenic mesentery, mesenteric fat, and small lymph nodes can be identified in the center of the intussusception (Fig. 51.19B,C). Trapped fluid has been associated with decreased success of nonsurgical reduction in a few studies (25,26). Color Doppler US has been utilized to assess the viability of the involved intestine (27,28) (Fig. 51.19D). Nonsurgical reduction should be attempted in any case with no evidence of free intraperitoneal air or clinical signs of peritonitis. The duration of symptoms and the presence of small bowel obstruction are not generally considered deterrents. Small amounts of free fluid are commonly seen with US and do not necessarily indicate perforation.
Enema reduction is performed using either water-soluble contrast or air under pressure. Air enema reduction has been favored by many pediatric radiologists in recent years because the procedure allows the generation of higher pressures that allow faster reduction time and improved reduction rate (29,30)
(Fig. 51.19E). Pressures must be monitored with a manometer during the procedure, and the intraluminal pressure should not exceed 120 mm of mercury. Hydrostatic reduction using water-soluble contrast material remains a viable alternative to air reduction. The contrast column needs to be elevated to a height of 4 to 5 feet of water-soluble contrast to generate comparable pressures to the air pumps. When appropriate pressures are generated with either procedure, reduction rates are in the range of 80% to 90%. US-guided hydrostatic or air reduction is gaining popularity worldwide (31,32,33,34,35,36).
(Fig. 51.19E). Pressures must be monitored with a manometer during the procedure, and the intraluminal pressure should not exceed 120 mm of mercury. Hydrostatic reduction using water-soluble contrast material remains a viable alternative to air reduction. The contrast column needs to be elevated to a height of 4 to 5 feet of water-soluble contrast to generate comparable pressures to the air pumps. When appropriate pressures are generated with either procedure, reduction rates are in the range of 80% to 90%. US-guided hydrostatic or air reduction is gaining popularity worldwide (31,32,33,34,35,36).
When intussusception is initially refractory to reduction, repeated attempts are often helpful (37). Avoiding the use of
sedation improves reduction rates by permitting the patients to increase intra-abdominal pressure via Valsalva maneuver (38). Recurrent intussusception occurs in approximately 5% to 10% of cases. When repeated occurrences of intussusception occur in a given patient, the possibility of a fixed lead point is increased and surgery should be considered.
sedation improves reduction rates by permitting the patients to increase intra-abdominal pressure via Valsalva maneuver (38). Recurrent intussusception occurs in approximately 5% to 10% of cases. When repeated occurrences of intussusception occur in a given patient, the possibility of a fixed lead point is increased and surgery should be considered.
Transient, spontaneously reducing intussusceptions are an increasingly common finding as the use of abdominal CT and US becomes more prevalent. Transient intussusception most commonly involves the small bowel. The intussusception is typically short in length, slightly echogenic at US, and smaller in diameter than the typical ileocolic intussusception (39,40) (Fig. 51.20). Intussusception can also occur along the course of a gastrojejunostomy tube (41).
Appendicitis. After approximately 2 years of age, perforated appendicitis becomes an increasingly common cause of an obstructive pattern on radiographs. Radiographs tend to be normal or show diminished intestinal gas with acute nonperforated appendicitis. After appendiceal rupture occurs, small bowel dilatation gradually develops because of a combination of decreased small bowel peristalsis and partial bowel obstruction in the region of abscess formation. Symptoms may temporarily
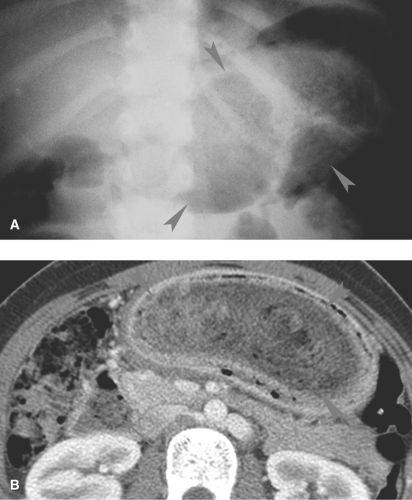
improve following perforation because of the intense inflammatory response that occurs around the appendiceal perforation. Free intra-abdominal air may be detected on CT but is rarely seen radiographically. As an abscess develops, radiographs may show a right lower quadrant mass. Once the appendix perforates, obstruction gradually develops due to a combination of functional obstruction and abscess formation (Fig. 51.21A).
improve following perforation because of the intense inflammatory response that occurs around the appendiceal perforation. Free intra-abdominal air may be detected on CT but is rarely seen radiographically. As an abscess develops, radiographs may show a right lower quadrant mass. Once the appendix perforates, obstruction gradually develops due to a combination of functional obstruction and abscess formation (Fig. 51.21A).
Abscesses are best detected with US or CT (Fig. 51.21B,C). Sonographically, appendiceal abscesses vary from anechoic to solid in appearance. The appendix can be difficult to identify with US after perforation, but other findings such as abnormal fluid collections, mesenteric edema, and absent peristalsis in local bowel loops can suggest the diagnosis (Fig. 51.21D).
Regional enteritis patients clinically mimic those with chronic appendiceal abscess. US shows the thickened wall of the involved small bowel, but contrast studies better evaluate the extent of involvement and provide the definitive findings. Other diseases, such as tuberculosis, lymphoma, and Yersinia colitis, are much less common but produce findings that may be difficult to differentiate from regional enteritis.
Table 51.6 Causes of Colonic Obstruction | |||||||||
|---|---|---|---|---|---|---|---|---|---|
|
Colonic Obstruction.
Congenital obstructions of the colon are more common than acquired obstructions (Table 51.6).
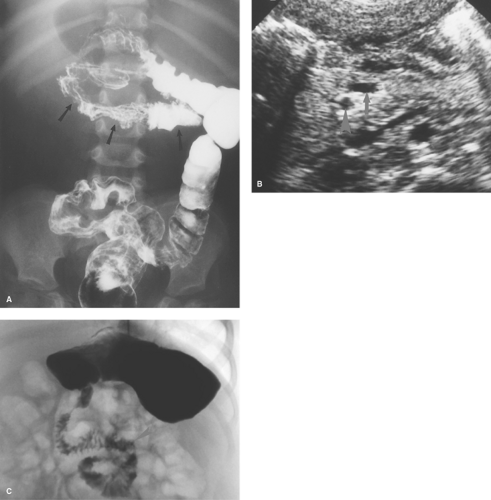
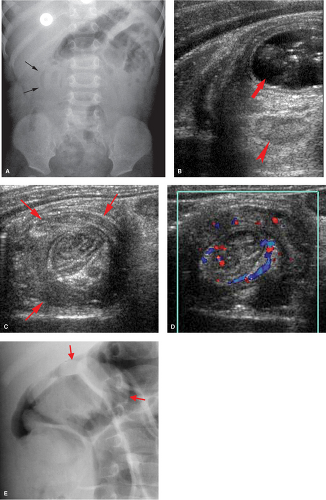
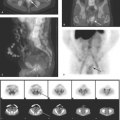
Hirschsprung disease is the result of absence of ganglion cells Auerbach and Meissner plexuses of the distal colon and rectum, resulting in abnormal peristalsis and inability to effectively evacuate the colon. The rectum is always involved, but the extent of proximal involvement varies. The aganglionic segment is characteristically contracted and smaller in caliber than the proximal normal colon. A well-defined change in caliber at the zone of transition is characteristic in older infants (Fig. 51.22A) but is frequently not present in neonates (Fig. 51.22B). Spasticity or corrugation of the narrowed aganglionic segment
of the colon is commonly seen (Fig. 51.22C,D). Evacuation of contrast is delayed, usually well beyond 24 hours. Diagnosis of Hirschsprung disease is definitively made with rectal biopsy. Necrotizing enterocolitis (NEC) is an uncommon but serious complication of Hirschsprung disease due to stasis colitis.
of the colon is commonly seen (Fig. 51.22C,D). Evacuation of contrast is delayed, usually well beyond 24 hours. Diagnosis of Hirschsprung disease is definitively made with rectal biopsy. Necrotizing enterocolitis (NEC) is an uncommon but serious complication of Hirschsprung disease due to stasis colitis.
Total colon aganglionosis is a rare form of Hirschsprung disease. Lack of ganglion cells can extend into the small bowel. The colon can appear normal in caliber or diffusely small (microcolon). A question-mark configuration has been described, consisting of a dilated cecum and ascending colon, gradually decreasing caliber toward the rectum (42). The diagnosis of total colonic aganglionosis can be difficult and is often suggested by the persistence of symptoms after other causes of colonic obstruction have been excluded.
Functional megacolon is a common condition in childhood that is associated with spasm of the puborectalis muscle. In
many instances, the muscle spasm is secondary to anal fissures; in other cases it is idiopathic and probably multifactorial. The rectum is normal to large in caliber. Prominence of the puborectalis sling provides the major clue to diagnosis. These patients can hold considerable volumes of stool in their colon.
many instances, the muscle spasm is secondary to anal fissures; in other cases it is idiopathic and probably multifactorial. The rectum is normal to large in caliber. Prominence of the puborectalis sling provides the major clue to diagnosis. These patients can hold considerable volumes of stool in their colon.
Anorectal malformations are common causes of distal bowel obstruction in the neonate. Anatomic deficits range from simple membranous anal atresia to abnormal descent of the colon through the puborectalis sling, with fistula formation from the blind-ending rectal pouch to some part of the genital or urinary tract (Fig. 51.23). In females, the fistula may empty into the bladder, uterus, or vagina. In males, it tends to enter into the urethra or bladder. In both sexes, it can enter the perineum. Sacral and urinary tract anomalies, hydrometrocolpos, and persistent cloaca are associated. When sacral abnormalities are present, the spinal cord and canal should be screened with US for abnormalities such as cord tethering or masses (43). Virtually all of these patients have neurogenic bladder dysfunction (44).
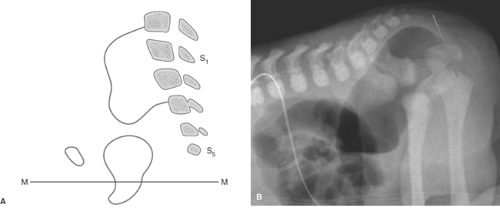
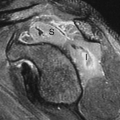
The goal of imaging is to identify associated anomalies as well as the location of the fistula. US can demonstrate the distal end of the pouch. Using a perineal approach, the distance between the anal dimple and the end of the rectal pouch can be measured. The distance is less than 1.5 cm with low lesions. The fistula may be injected directly or may opacify during retrograde voiding cystourethrography. In females, flush retrograde vaginography may be required. If the fistula empties above the puborectalis sling, it can be presumed that the puborectalis muscle is hypoplastic and that continence will be difficult to accomplish with any surgical procedure. If the fistula empties below the puborectalis sling, the puborectalis muscle is usually more developed and achieving continence is more likely. The “M” line of Cremin has been utilized to determine the level at which the blind pouch ends on lateral radiographs (Fig. 51.24). This line is drawn perpendicular to the long axis of the ischia on lateral view and passes through the junction between the middle and lower third of the ischia. If the blind pouch and fistula end above the line, the fistula is considered high. If they end below the line, it is considered low, and if they end at the line, it is considered intermediate. Intermediate fistulae usually pose the same problems as high fistulae. MRI is useful in some patients to evaluate the adequacy of the sphincter muscle complex, assess the position of the fistula, and identify the associated masses or genitourinary anomalies (45). Postoperatively, MRI can establish that the pull-through is properly positioned in relation to the sphincter (46).
Colon atresia is relatively rare and is evident in the neonatal period. Massive distension of the colon is seen proximal to the area of atresia or stenosis (Fig. 51.25).
Acquired causes of colonic obstruction are relatively uncommon except for perforated appendicitis and regional enteritis. Inflammatory strictures associated with ulcerative colitis and necrotizing enterocolitis tend to be smooth and appear similar to those in adults. They may be single or multiple (Fig. 51.26). Colonic strictures can also be found in children with cystic fibrosis (20,47). Tumors of the colon are uncommon, and the findings are similar to those seen in adults. Trauma to the colon, producing obstruction, is seen with motor vehicle accidents or, more commonly, the battered child syndrome. Sigmoid and cecal volvulus are much less common in children than in adults, but the imaging findings are the same. Volvulus occurs more frequently in bedridden patients and in neurologically impaired children.
Inflammation and Infection
Gastrointestinal Tract.
Esophagitis in children is commonly caused by peptic disease, caustic ingestion, viral and monilial infection. Peptic esophagitis tends to involve the lower third of the esophagus but, with severe reflux, may involve the entire esophagus. Findings consist of thickening of the esophageal wall, lack of normal peristalsis, tertiary contractions in the esophagus, and ulcers that may be superficial or deep. Incomplete relaxation of the cricopharyngeal muscle during swallowing can be a clue to the presence of GER on swallowing studies. Caustic ingestion and extensive esophageal burns are the rule. Perforation into the mediastinum can occur. Contrast studies generally are not performed acutely following caustic ingestion, but such studies are useful later to demonstrate strictures (see Fig. 51.4). Viral esophagitis (e.g., herpes) causes small superficial ulcers, best demonstrated with double-contrast studies. The ulcers can be diffuse or focal and may result in intense spasm with severe dysphagia. Monilial esophagitis causes mucosal irregularities with intense spasm of the esophagus leading to pseudodiverticula.
Gastritis is commonly caused by peptic disease or helicobacter and campylobacter infection in infants and children. The findings resemble those seen in adults; superficial ulcerations and delicate, edematous, cobblestone appearance of the mucosa can occur. Milk allergy is a common cause of gastritis in infants that leads to vomiting and bleeding. Upper GI series in such infants may demonstrate intense antropyloric spasm. US may show thickening of the mucosa in any cause of gastritis. Prominent mucosal inflammation and thickening may lead to gastric outlet obstruction in children with chronic granulomatous disease.
Duodenitis is usually caused by peptic disease which is more common in children than is often recognized. Penetrating ulcers are sometimes seen. Children tend to present with bleeding more often than adults.
Gastroenteritis is most often caused by viral infections in children. US shows distended fluid-filled loops with thickened
mucosa (Fig. 51.27). Abdominal radiographs may show extensive gaseous distention of the GI tract that may erroneously suggest mechanical obstruction. Thickening of the small bowel mucosa can also occur with milk allergy and bacterial infections such as Yersinia, Campylobacter, and Salmonella. Tuberculosis should be considered in regions where the infection is endemic. Eosinophilic enteritis, celiac disease, and lymphangiectasia are rare causes of small bowel thickening in childhood.
mucosa (Fig. 51.27). Abdominal radiographs may show extensive gaseous distention of the GI tract that may erroneously suggest mechanical obstruction. Thickening of the small bowel mucosa can also occur with milk allergy and bacterial infections such as Yersinia, Campylobacter, and Salmonella. Tuberculosis should be considered in regions where the infection is endemic. Eosinophilic enteritis, celiac disease, and lymphangiectasia are rare causes of small bowel thickening in childhood.
Regional enteritis (Crohn disease) usually affects the terminal ileum as the primary site of involvement. Regional enteritis of the proximal small bowel, stomach, or esophagus is uncommon. Contrast small bowel fluoroscopic examinations remain the most common method for evaluating small bowel involvement. The radiographic findings are identical to those in adults and consist of variable lengths of narrowed irregular terminal ileum, with or without linear ulcers and sinus tracts (Fig. 51.28A,B). The transmural bowel wall thickening common in these patients is readily demonstrable with US (48) (Fig. 51.28C). Loss of normal bowel wall stratification and decreased peristalsis are also seen on US. Color Doppler US reveals increased vascularity in involved intestinal loops (49) (Fig. 51.28D) and can be used to follow the effectiveness of therapy (50). Lack of bowel wall thickening on US has a strong negative predictive value when performed by experienced sonographers (51). MDCT is primarily used to evaluate acute complications such as abscess, perforation, or postsurgical leaks. Gadolinium-enhanced MRI is the best modality for the evaluation of perianal disease, fistulae, and abscesses (52,53). MR and CT enterography are promising techniques that provide better bowel distention and may help to distinguish active bowel disease from fibrosis (54,55,56). It should be noted that patients who develop Crohn disease during childhood are at risk of being exposed to high levels of radiation during radiological evaluations in their lifetime, which could result in radiation-induced cancer (57,58).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree