Congenital lung malformations are a spectrum of developmental anomalies comprised of malformations of the lung parenchyma, airways, and vasculature. Imaging assessment plays a pivotal role in the initial diagnosis, management, and follow-up evaluation of congenital lung malformations in the pediatric population. However, there is currently a lack of practical imaging guidelines and recommendations for the diagnostic imaging assessment of congenital lung malformations in infants and children. This article reviews the current evidence regarding the imaging evaluation of congenital lung malformations and provides up-to-date imaging recommendations for pediatric congenital lung malformations.
Key points
- •
Pediatric congenital lung malformations (CLMs) comprise a spectrum of developmental anomalies of the lung parenchyma, airways, and vasculature. CLMs are increasingly diagnosed prenatally, but remain best characterized by postnatal cross-sectional imaging.
- •
Management of CLMs, including imaging, in infants and young children depends on associated symptoms and institutional standards.
- •
Chest CT angiography (CTA) performed not before 4 weeks of age is usually the most appropriate initial postnatal imaging modality for assessing prenatally diagnosed or clinically suspected CLMs in asymptomatic infants and children.
- •
Chest magnetic resonance (MR) imaging / magnetic resonance angiography (MRA) may be considered as a complementary, problem-solving, or follow-up imaging modality for evaluation of CLMs.
Introduction
Congenital lung malformations (CLMs) comprise a spectrum of developmental anomalies including malformations of the lung parenchyma, airways, and vasculature. Although the historical incidence of CLMs is 1 in 10,000 to 35,000, , CLMs are being increasingly detected, especially prenatally, with an apparently increasing incidence, recently as frequent as 4 in 10,000 births. This apparent increase in the incidence of CLMs is at least partially caused by recent improvements in and increased use of fetal ultrasound and fetal magnetic resonance (MR) imaging. Over the past three decades, this increased incidence and detection of CLMs has aided the understanding of the imaging features of various types of CLMs, their potential etiologies, and the natural history of these anomalies.
Diagnostic imaging evaluation plays a pivotal role in the initial diagnosis, preoperative planning, and follow-up evaluation of CLMs in the pediatric population. Unfortunately, there is currently a paucity of practical imaging guidelines and recommendations for the imaging assessment of CLMs in infants and children. Therefore, the purpose of this article is to review the current imaging modalities and techniques for evaluating CLMs postnatally, and provide up-to-date imaging guidelines and recommendations for practicing radiologists and clinicians managing pediatric patients with CLMs.
Evidence-based imaging algorithm
Table 1 provides a brief summary of the findings of selected previously published articles, comparing the currently available imaging modalities for evaluating CLMs postnatally.
| Imaging Modality | Sensitivity | Specificity | Pathology Concordance | Advantages | Disadvantages | References |
|---|---|---|---|---|---|---|
| Radiography | 50%–61% | N/A | N/A | Wide availability Low cost | Low sensitivity Limited characterization Radiation (low) | , |
| Ultrasound | N/A | N/A | N/A | Wide availability Less costly No radiation | Operator dependent | |
| Chest CTA with contrast | 85%–100% | 75%–100% | 83.5% | Best spatial resolution Best lung parenchyma evaluation Best feeding vessel evaluation Wide availability | Radiation Limited in patients with suboptimal renal function and allergy to contrast More expensive than radiography and ultrasound Possible small risk from iodinated contrast use | , , , |
| Chest MR imaging without contrast | 57% | 100% | 96% | Best soft tissue characterization No radiation | Longer examination often requiring sedation Susceptibility artifact from air cant limit evaluation of lung parenchyma Costly | , , |
| Chest MR imaging/MR angiography with contrast | 96% | Best soft tissue characterization No radiation Able to delineate feeding vessel | Longer examination requiring sedation Susceptibility artifact from air limits evaluation of lung parenchyma Costly Possible small risks from gadolinium use |
In the prenatal period, fetal ultrasound and fetal MR imaging have high sensitivity for detecting CLMs; however, imaging characterization of CLMs with fetal ultrasound and fetal MR imaging is often incomplete, especially for solid lung parenchymal CLMs. For example, Mon and colleagues found that prenatal ultrasound had an accuracy of 72% and fetal MR imaging had slightly higher accuracy of 80% for correctly identifying an aberrant systemic vessel in a series of 103 parenchymal CLMs that were found be 25% pulmonary sequestration, 44% congenital pulmonary airway malformation (CPAM), and 21% CPAM/sequestration hybrid lesions at the time of surgical excision. In this same study, postnatal chest computed tomography angiography (CTA) had a superior accuracy of 90% for identifying the systemic feeding vessel in these CLMs. The presence of a systemic feeding vessel is an important diagnostic feature for distinguishing CLMs, especially when differentiating CPAM from pulmonary sequestration. In this study, approximately 25% of CLMs diagnosed by prenatal ultrasound were found to have a different pathology at the time of surgical excision, likely at least partially caused by missing the systemic feeding vessel. Of note, the opposite finding was noted by Beydon and colleagues who found that fetal ultrasound had higher systemic vessel detection than fetal MR imaging, although this was a smaller study (n = 23). Lastly, prenatal imaging has reduced sensitivity for lesions whose predominant imaging features relate to aeration of the lung, such as congenital lobar overinflation (CLO). Collectively, these studies and others continue to demonstrate that prenatal ultrasound and fetal MR imaging can provide important information early, but have reduced sensitivity and specificity for accurately diagnosing CLMs compared with postnatal chest CTA.
Although the use of chest CTA for assessing CLMs has been investigated more thoroughly, MR imaging of the chest is now being evaluated as a possible postnatal imaging alternative to chest CTA. Historically, MR imaging of the chest had a specific complementary role in assessing vascular CLMs, allowing for assessment of cardiac blood flow and shunt vascularity. More recently, MR imaging of the chest has been used to assess parenchymal CLMs. For example, in a series of 23 cases, Zirpoli and colleagues compared chest MR imaging without contrast with chest CTA and found comparable sensitivity and specificity between the two modalities except in regards to aberrant vessel detection. In addition, Kellenberger and colleagues recently found that postnatal chest MR imaging with contrast correctly diagnosed 96% of abnormalities (n = 46), including aberrant vessel detection, when compared with surgical pathology or other gold standard imaging modalities, such as echocardiogram and computed tomography (CT). Although direct comparison between postnatal CT and postnatal MR imaging was only available in 33% (n = 13) of these cases, 100% concordance was noted in these 13 cases. All of the CLMs in the study demonstrated reduced perfusion compared with normal lung parenchyma at peak pulmonary enhancement, a sensitive imaging feature on MR imaging that is not assessed by CTA. Newman recently described typical scenarios where MR imaging is being used in place of CTA for CLMs, including: (1) follow-up of a known CLM, defined previously either by prenatal MR imaging or prior CTA, if repeat radiation exposure is of concern; (2) to define a mass that might not initially be suspected to be a CLM if present in a less common location, such as paraspinal, mediastinal, or intra-abdominal; (3) as a supplemental imaging modality if there is incomplete characterization of the CLM by CTA, often in the case of complex lesions; and (4) incidentally noted CLMs on an MR imaging obtained for other reasons, such as cardiac or abdominal pathology.
Despite these early optimistic findings demonstrating the utility of chest MR imaging for postnatal CLM evaluation, CTA remains the first choice for characterizing CLM for several reasons. First, CTA is more readily available and a less expensive modality than MR imaging. Second, although sedation can sometimes be avoided in infants obtaining MR imaging (using feed and wrap protocol) and occasionally is needed for CTA, of the two examinations, CTA can more often be performed without sedation. Additionally, if sedation is to be used, it often is quicker and with lesser anesthetic depth for chest CTA than with chest MR imaging. A recently published study showed that sedation can often be avoided in the assessment of CLMs in infants and young children (≤6 years old) using the newer dual-source CT imaging without compromise of the diagnostic quality. Avoiding sedation in young children is important because animal studies have indicated there is a possible small risk of neurotoxicity with the use of anesthetics in neonatal and young pediatric patients. For this reason, the Food and Drug administration has placed warning labels on several sedative medications, such as propofol, ketamine, and lorazepam, stating that “exposure to these agents for lengthy periods of time or over multiple procedures may negatively affect brain development in children younger than 3 years.”
Third, the contrast agents used for both examinations have slightly different risks associated with them. Iodinated contrast agents are generally well tolerated with the main concern being a potential small risk of contrast-induced nephropathy, which has been previously reported to occur most commonly in a small percentage of adults with risk factors, such as underlying renal dysfunction, although this remains controversial. In the pediatric population, this association is even less well-delineated with a recent study by Gilligan and colleagues finding no evidence of contrast-induced nephropathy in hospitalized pediatric patients withstable renal function (glomerular filtration rate>60). Another recent study by McGaha and colleagues found no occurrence of contrast-induced nephropathy in injured pediatric patients undergoing CT scan for trauma assessment.
Gadolinium-based MR imaging contrast agents also have good safety profiles, with the main reported association being a small risk of nephrogenic systemic fibrosis, which most often occurs in the setting of renal dysfunction. Although there are reported cases of nephrogenic systemic fibrosis in the pediatric population, the rate of nephrogenic systemic fibrosis has significantly decreased over the last decade with the use of better, lower risk gadolinium contrast agents and restriction of use with renal dysfunction. An additional significant concern related to the use of gadolinium contrast agents is that several studies have indicated that use of these agents may result in gadolinium deposition in tissues, such as the brain, typically after several MR imaging studies with contrast. No studies have been able to show any clinically significant outcome from this gadolinium deposition; however, long-term studies are needed to assess whether there are clinical effects of this deposition.
These advantages and disadvantages of iodinated and gadolinium-based contrast agents are important considerations that may factor into the decision to use CTA or MR imaging for the assessment of CLMs. Whatever imaging modality is selected, it is of outmost importance that a good-quality, angiographic study be obtained to adequately depict the presence of systemic feeding vessels.
Congenital lung malformation imaging algorithm
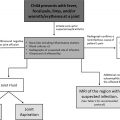
A summary of the postnatal imaging algorithm for the evaluation of CLMs is described in Fig. 1 . Postnatal suspicion for a CLM may either arise secondary to a concern on prenatal imaging or from clinical symptoms, such as respiratory distress, tachypnea, cough, wheeze, or recurrent infections, either in an infant or child. Prenatal imaging alone is not sufficient for the evaluation and characterization of a CLM postnatally because of the limitations of prenatal imaging and the possible evolution (and even regression) of CLMs during pregnancy and birth. CLMs may regress and/or display different imaging characteristics on postnatal imaging after the physiologic changes of parturition. For example, postnatal inflation of the lungs significantly changes the imaging appearance of CLO. The optimal postnatal imaging algorithm for a CLM depends on careful consideration of three main aspects that interplay into the final patient management and clinical decision making: (1) institutional surgical standards, (2) imaging study types, and (3) symptom assessment.

Institutional Surgical Standards
Institutional surgical standards affect the postnatal imaging algorithm of pediatric patients with CLMs. Currently, most pediatric hospitals in the United States advocate cross-sectional imaging evaluation in symptomatic and asymptomatic infants and children with a suspected CLM based on prenatal imaging findings and/or postnatal symptoms. This preference for imaging symptomatic and asymptomatic children in the United States is because of a prevailing belief that asymptomatic CLMs can cause future symptoms, such as recurrent infections, and have a small reported risk of malignancy, leading many American institutions to recommend surgical intervention, even in the case of currently asymptomatic CLMs. At other institutions, particularly in Europe, alternative imaging and management strategies exist for CLMs, with some recommending watchful waiting and only performing cross-sectional imaging in those children with a suspected CLM that develops symptoms. At these institutions, surgical intervention is only performed if the patient becomes symptomatic. Of note, asymptomatic CLMs are thought to become symptomatic in at least 30% of cases. Consequently, at these institutions, cross-sectional imaging is not performed in asymptomatic infants and children who are suspected of having a CLM because it would not change management. Therefore, careful consideration of each institution’s clinical standards toward CLM management is important for determining the postnatal imaging algorithm of pediatric patients with CLMs.
Types of Imaging Study
The second consideration in the imaging evaluation of a CLM is the type of imaging study. The most noninvasive and inexpensive screening modalities, radiography and ultrasound, are limited for the postnatal characterization of CLMs. Chest radiographs are often used as an initial imaging study; however, they lack sensitivity, specificity, and spatial resolution for the detection and characterization of CLMs. , Similarly, although ultrasound is used to characterize some CLMs and can demonstrate feeding vessels, it is challenging because air within the inflated lungs limits sonographic penetration and assessment.
In contrast, cross-sectional imaging modalities, specifically chest CTA and chest MR imaging, have high clinical utility in the postnatal assessment of CLMs. Both of these imaging modalities provide detailed anatomic information with substantial technical improvements in pediatric CT and MR imaging techniques over the past twodecades. Chest CTA still remains the preferred imaging modality for evaluating CLMs, mainly because of its ability to completely evaluate lung parenchyma and identify associated subtle vascular abnormalities. , , However, exposure to potentially harmful ionizing radiation and the need for sedation remains an important concern in infants and children. Fortunately, recent improvements in CT technologies have led to substantial reductions in radiation dose and faster scanning speeds, decreasing the need for sedation.
Chest MR imaging remains an important, radiation-free imaging alternative to chest CTA. Similar to ultrasound, air within the lungs can cause substantial artifact and signal void on MR imaging, slightly decreasing its sensitivity for the detection of small CLMs within the aerated lungs. In addition, respiratory motion can also cause substantial motion artifact on chest MR imaging, further limiting evaluation. However, newer faster MR imaging sequences, such as steady-state acquisition and single-shot fast spin echo, can decrease motion artifact and also provide improved soft tissue characterization of CLMs. , If intravenous (IV) contrast is administered, chest MR imaging can also characterize the vasculature within CLMs. Furthermore, because parenchymal CLMs have been reported to have reduced enhancement compared with normal lung parenchyma, contrast sequestration at peak perfusion is useful for delineating CLMs.
Symptom Assessment
Clinical symptoms also play an important role in determining the imaging approach for pediatric patients with a CLM. If severe respiratory distress is present perinatally, emergent postnatal cross-sectional imaging is typically performed to expedite diagnosis and aid in surgical planning. For pediatric patients with milder symptoms, such as cough, wheezing, or recurrent respiratory infections,with clinical concern for an underlying CLM, screening chest radiography may be performed initially, followed by cross-sectional imaging as close as possible to an appropriate age for surgical intervention. For example, in infants with mild symptoms, surgical intervention is typically preferred around 6 to 12 months after the development of active humoral immunity but before recurrent respiratory infections that could result in scarring or complicate surgical resection.
Practical imaging approach to pediatric congenital lung malformations
The technical details of the currently available imaging modalities for evaluating CLMs in infants and children are beyond the scope of this article. The following section summarizes the utility of various imaging modalities for the detection of various CLMs, including characteristic postnatal imaging features.
Radiography
Radiography is often used as an initial screening imaging modality for detection of a CLM after birth. On chest radiographs, persistent opacities or lucencies, and recurrent infections in the same location (especially the left lower lobe for sequestration), raise suspicion for an underlying CLM. However, radiographs are insensitive for the detection of CLMs. Specifically, the lack of a visible abnormality on chest radiograph does not exclude a CLM. If there is strong clinical concern for a CLM, further evaluation with cross-sectional imaging is warranted for complete assessment.
Ultrasound
Ultrasound has limited utility for the postnatal detection of CLMs secondary to reduced penetration of sound waves through aerated lung (although it has been used in the past before improvements in other modalities). On ultrasound, a CLM may appear as a well-circumscribed focal lung abnormality with cystic, solid or mixed components, with or without associated abnormal vessels (best assessed with Doppler ultrasound imaging). Of note, a recent case report described the use of contrast-enhanced ultrasound to guide biopsy of a CPAM in an adult. Use of contrast-enhanced ultrasound for this purpose in infants and children has yet to be fully evaluated.
Computed Tomography Angiography
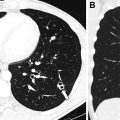
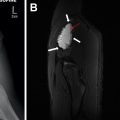
With the highest spatial resolution and sensitivity for the postnatal detection of CLMs, chest CTA is the current gold standard for postnatal evaluation of CLMs. To maximize detection of abnormal vasculature often associated with CLMs, chest CTA technique is recommended, with the anatomic coverage extending from the lower neck to the mid-abdomen to fully capture the extent of any abnormal vasculature, which may arise or extend below the diaphragm. The need for sedation depends on patient age and ability to cooperate with breathing instructions; however, many chest CTA studies are able to be successfully completed within the first year of life without sedation. Our recent study, which directly compared the diagnostic quality of chest CTA without and with general anesthesia in appropriately screened infants and young children for the evaluation of congenital thoracic disorders using multidetector CT with turbo flash spiral mode and free-breathing technique, showed that only a small minority (3%) of chest CTA studies performed without general anesthesia had motion artifact severe enough to limit evaluation. The results of this study support the use of chest CTA without general anesthesia (using turbo flash spiral mode and free-breathing technique) for detecting and evaluating CLMs.
In the authors’ experience, in asymptomatic children, it is desirable not to image infants during the early neonatal period because delayed resorption of the expected fetal pulmonary fluid is challenging to distinguish from fluid-filled CPAM cysts.Furthermore, in cases of CLO, the expected hyperinflated lung distal to the abnormal bronchus may remain fluid-filled, not yet manifesting the characteristic hyperinflation.
Chest CTA post-processing techniques, such as multiplanar reformats and three-dimensional reconstructions of the airway and vasculature, are critical for the accurate characterization of CLMs. Lee and colleagues showed that although axial multidetector CT images allow accurate diagnosis of CLM type, location, mass effect, and anomalous arteries of CLMs, supplemental multiplanar reformats and three-dimensional reconstructions of the vasculature add significant diagnostic value for the evaluations of CLMs with increased detection of anomalous vessels, a potentially important finding for surgical planning. Therefore, post-processing multiplanar (two-dimensional) reformats and three-dimensional reconstructions are routinely performed for evaluation of CLMs in the pediatric population.
MR Imaging
MR imaging is an attractive, alternative cross-sectional imaging modality, especially in light of newer faster sequences that allow improved detection of CLMs within the aerated lungs, such as steady-state acquisition and single-shot fast spin echo. MR imaging can often be performed without sedation during at least the first 6 months of life and in older children who can follow breathing instruction. For infants and young children between these two age groups, sedation may be necessary because of the length of a typical chest MR imaging study. Similar to CTA, chest MR imaging field of view typically extends from lower neck through mid-abdomen, with acquisitions performed in three planes, usually with IV contrast. Kellenberger and colleagues recently found thatchest MR imaging with contrast, including peak perfusion enhancement, was useful for deciphering the extent of CLMs. In addition, for CLMs with abnormal vasculature, MR imaging of the chest can be obtained while undergoing simultaneous cardiac MR imaging to assess for flow and shunting.
It has been shown that MR imaging has good sensitivity for accurately detecting soft tissue and fluid components within larger CLMs and is useful for surgical planning. , Typical MR imaging features of CLMs are described next and differ depending on the CLM type. However, Kellenberger and colleagues described a useful imaging feature that all parenchymal CLMs demonstrated reduced enhancement at peak pulmonary perfusion after IV contrast administration.
Spectrum of pediatric congenital lung malformations
Although this review focuses on imaging recommendations and guidelines for evaluation of CLMs,which does not substantially change depending on the suspected type of CLM (because standard protocols should allow for diagnostic assessment of all CLM types), the following section briefly describes the salient imaging features of commonly encountered CLMs in children. In brief, for all of these CLMs, there are several imaging features that should be evaluated in all of these lesions for diagnosis and for surgical planning. These include the vascular supply and drainage of the CLM, the size of CLM, presence of aeration in the CLM (which typically occurs after 6–8 weeks of life), the presence of associated airway or bronchocele, and abnormal fissural anatomy (which plays an important role in surgical planning).
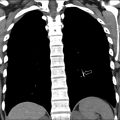
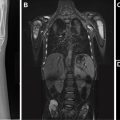
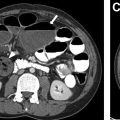
Congenital Foregut Duplication Cyst
Congenital foregut duplication cysts comprise three main different types of malformations including bronchogenic, esophageal, and neurenteric duplication cysts ( Fig. 2 ). During the first 4 weeks of gestation, the tracheal buds form from the ventral aspect of the primitive foregut. , Foregut duplication cysts are thought to derive from abnormal budding, either in this initial step or possibly later within subsequent development of the branches of the airway. In keeping with the hypothesis of abnormal budding, esophageal duplications cysts typically occur near or within the esophagus, and bronchogenic duplication cysts occur somewhere within or near the airway. The esophagus is the second most common location for enteric duplication cysts after the ileum. Foregut duplication cysts, specifically neurenteric cysts, may be associated with Klippel-Feil syndrome and hemivertebrae. , Affected pediatric patients may present with dysphagia from mass effect on the esophagus and/or respiratory symptoms because of mass effect on adjacent airways.