High-field 3 T magnetic resonance (MR) imaging provides greater signal-to-noise ratio (SNR) compared with 1.5 T systems. Various MR imaging clinical applications in children can benefit from improvements resulting from this increased SNR. High-resolution imaging of the brain, arterial spin labeling perfusion imaging, diffusion imaging, MR spectroscopy, and imaging of small anatomic parts are some areas in which these improvements can increase our clinical diagnostic capabilities. However, challenges inherent to 3 T imaging become more relevant in children. The use of 3 T imaging in children has allowed better diagnostic efficacy in neuroimaging, but certain technique modifications may be required for optimal imaging.
- •
Most of the added value of using high-field 3 T magnetic resonance imaging derives from being able to take advantage of the spatial and temporal benefits of multichannel coil technology and parallel imaging without unacceptable signal loss.
- •
Increased spatial resolution is highly desirable or sometimes necessary in pediatrics to delineate small structures and subtle disease especially in younger children, and 3 T high-field imaging can be effectively used toward this goal.
- •
Using the advantages of high-field 3 T imaging in achieving faster scan times can help reduce the need for sedation or anesthesia, although in practice the signal gain of 3 T high-field imaging is more commonly exploited to increase spatial resolution and image quality than to decrease scan time.
- •
Many medical devices that have been declared safe for use on 1.5 T systems may not be safe using higher field magnets.
- •
The need for a smaller field of view (FOV) in pediatric imaging results in decreased signal-to-noise ratio (SNR). The increased SNR associated with the use of 3 T magnetic resonance (MR) imaging compensates for the loss of SNR and allows small FOV imaging with adequate image quality.
- •
The small head of the child should be placed in the center of the FOV when using multielement head coils to decrease image intensity inhomogeneity or shading.
- •
Specific absorption rate limits have to be taken into consideration when scanning children, particularly small infants who are kept warm in the scanner.
- •
Susceptibility effects are increased at 3 T. Modification of imaging techniques may be necessary to reduce susceptibility in patients, especially those with metallic implant and devices.
- •
The smaller FOV and better SNR makes 3 T favored for delineating small parts such as the temporomandibular joints, inner ear structures, and cranial nerves.
- •
In patients with epilepsy, the higher spatial resolution possible with the improved SNR makes 3 T imaging favored for detecting small malformations and epileptogenic foci.
- •
The improved contrast-to-noise ratio and SNR provide better details in MR angiography.
- •
Stronger chemical shifts, increased SNR, and higher order shimming allow for improved spectral quality in MR spectroscopy.
- •
High-field imaging allows more robust arterial spin labeling perfusion imaging in the assessment of cerebral blood flow without the need for intravenous contrast administration.
- •
Optimization of 3 T imaging of the pediatric spine (particularly T1-weighted images) is necessary to provide adequate image quality.
This review addresses basic considerations for pediatric neuroimaging at 3 T MR imaging, highlights the many advantages of scanning at higher magnetic fields, and evaluates the impact of 3 T MR imaging on the clinical practice of pediatric neuroradiology, as well as pointing out the difficulties and opportunities faced during day-to-day clinical work.
Principles of high-field 3 T imaging as applied to pediatrics
Applying a higher magnetic field strength increases longitudinal magnetization and results in improvement of SNR. MR signal increases by a factor of 4 at 3 T compared with 1.5 T, but the noise also increases by a factor of 2. This situation should theoretically double the SNR at 3 T compared with 1.5 T, but, in practice, the SNR increase is mostly less than double. A combination of factors including sequence modifications as a result of SAR limits, coil design, B 0 and B 1 inhomogeneity, and certain imaging parameter alterations for maintaining good image contrast result in an SNR that is less than this promised factor of 2. For example, if the repetition time (TR) in a spin echo sequence is kept constant despite the longer T1 relaxation times of tissue at 3 T (see subsequent section), there is a T1-weighting reduction in SNR, confounding the increase derived from field strength. Increasing the TR to compensate for the longer T1 increases the acquisition time. The use of higher receiver bandwidth to reduce susceptibility effects also contributes to loss of SNR.
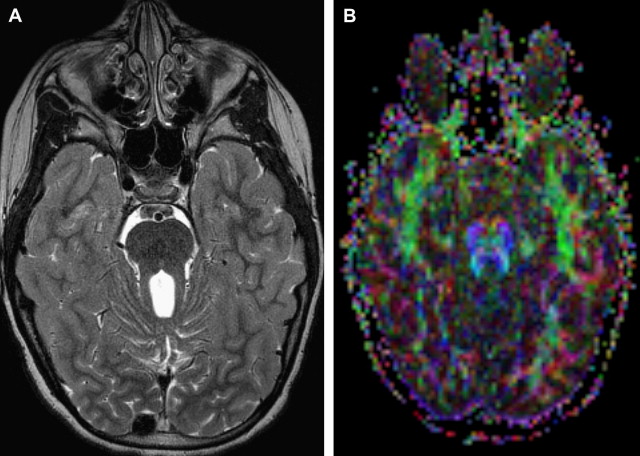
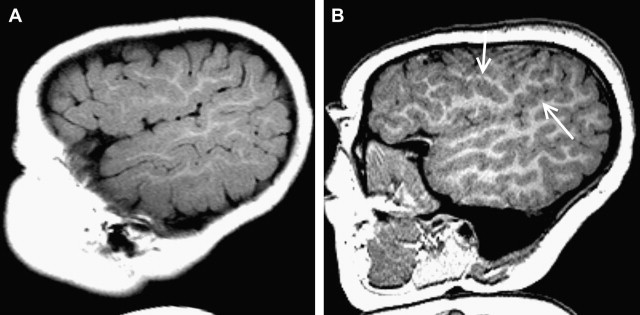
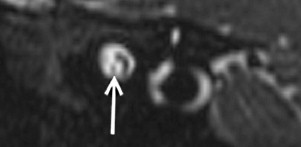
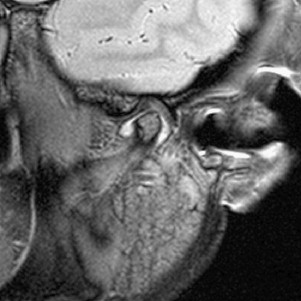
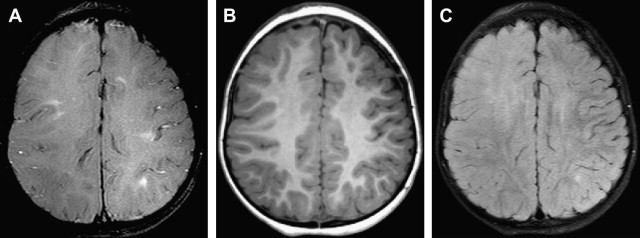
The SNR increase (even if <2 times) can be invested to substantially increase the spatial resolution, yielding better depiction of anatomic structures, and in turn, in principle, increase diagnostic confidence. Thinner slices can be acquired to improve spatial details of subtle abnormalities and small structures. Imaging of small structures is more problematic in children than in adults, especially in neonates and very young children. The better detail in brain lesions becomes relevant in malformations of cortical development, which can be challenging to diagnose and classify ( Fig. 1 ). The higher signal can also facilitate high-resolution imaging of small fluid-filled intracranial structures such as the inner ear and internal auditory canal (IAC). The higher resolution provides increased confidence in making diagnoses such as absence or thinning of cranial nerves, for example those within the IAC ( Fig. 2 ). The introduction of multichannel head coils has for the most part obviated use of small surface coils. The combination of high magnetic field and multichannel coils allows exquisite imaging of small articulations, which is exemplified in evaluations of the temporomandibular joints ( Fig. 3 ).
The SNR increase can alternatively be used to increase the temporal resolution, facilitated by reducing the number of needed averages to obtain a good-quality image. Theoretically, an SNR advantage of times 2 can be traded for up to a 4-fold increase in temporal resolution. By doing so, the acquisition time can be reduced and the same spatial resolution maintained. This situation is useful in children, who are typically more anxious and distracted, and hence move more frequently than adults during MR imaging examinations. The improved temporal resolution at higher magnetic field strengths coupled with motion-insensitive or motion-compensated sequences (eg, PROPELLER [periodically rotated overlapping parallel lines with enhanced reconstruction] and BLADE) contribute to the reduction of studies requiring pharmaceutical sedation. Sedation has to be performed by trained personnel, requires MR-compatible equipment for monitoring and rescue (if needed), is time-consuming, and carries potential inherent risks, particularly respiratory depression (albeit an uncommon occurrence).
| Routine Sequences a |
|---|
| T1 MPRAGE–sagittal 0.9-mm isotropic voxels reformatted into orthogonal planes |
| Axial two-dimensional (2D) turbo spin echo T2–2-mm thickness–384 × 384 matrix |
| Axial 2D FLAIR–4-mm thickness–320 × 320 matrix |
| Axial spin echo T1–4-mm thickness–256 × 256 matrix |
| Pulsed arterial spin labeling perfusion–8-mm thickness–64 × 64 matrix |
| Coronal or sagittal 2D fast spin echo T2–2-mm thickness–384 × 384 matrix–orientation depending on indication and if performed as part of a contrast brain study, performed immediately after gadolinium contrast injection |
| Diffusion–30 directions–2.5-mm thickness–128 × 128 matrix–b = 1000 |
| Optional Sequences Depending on Indication |
|---|
| Coronal 2D FLAIR–4-mm–320 × 320 matrix |
| MRA–3D TOF–0.5-m isotropic voxels |
| MRV–2D TOF–both axial and coronal acquisitions–3-mm thickness–256 × 256 matrix |
| GRE susceptibility–4-mm thickness–256 × 256 matrix |
| SWI–2-mm thickness–320 × 320 matrix |
| CISS–0.5-mm thickness–256 × 256 matrix–small FOV |
| Repeat T1 MPRAGE after contrast with same parameters as above, reconstructed in orthogonal planes |
| Repeat SE T1 after contrast with same parameters as above (patients receive both T1 MPRAGE and SE T1 after contrast) |
a Most are performed with GRAPPA (generalized autocalibrating partially parallel acquisition) 2 parallel acceleration.
Many of the clinical applications that were made possible by high-field imaging at 3 T are dependent on the ability to either generate images with better spatial resolution or perform quicker studies and maintain adequate spatial resolution. In our experience, and especially for the smaller field of view (FOV) required for pediatric applications, often the former is true and the increased signal is more commonly exploited to acquire higher spatial resolution images.
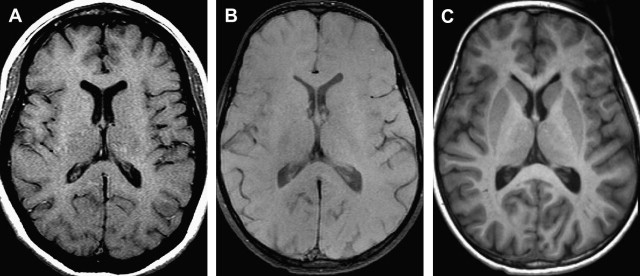
T1 relaxation times increase with increasing field strength, whereas the absolute differences between tissue T1 values become smaller. Therefore, the overall T1 contrast between tissues tends to decrease at higher magnetic fields ( Fig. 4 ). However, because the SNR is improved at higher magnetic fields, the contrast-to-noise ratio (CNR) is typically higher. The decreased T1-weighted contrast can be overcome by sequence change, for example using inversion recovery fast-segmented gradient sequences such as three-dimensional (3D) magnetization-prepared gradient echo (MPRAGE) and 3D fast magnetization-prepared spoiled gradient echo instead of spin echo sequences (see Fig. 4 ). These sequences offer superb gray-white matter contrast and have the additional advantage of being a 3D acquisition, which can be easily acquired in an isotropic or near isotropic fashion and later reformatted into any desired plane. The latter advantage can be helpful in reducing overall scanning times (by eliminating extra sequences showing different planes), which is of particular importance in pediatric imaging. The sharp gray-white differentiation is helpful in identifying subtle areas or delineating the full extent of malformations, such as those of cortical development.
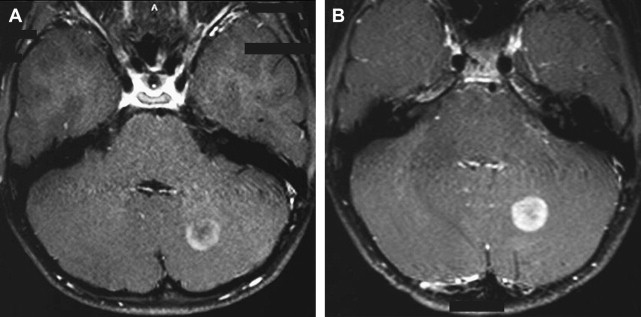
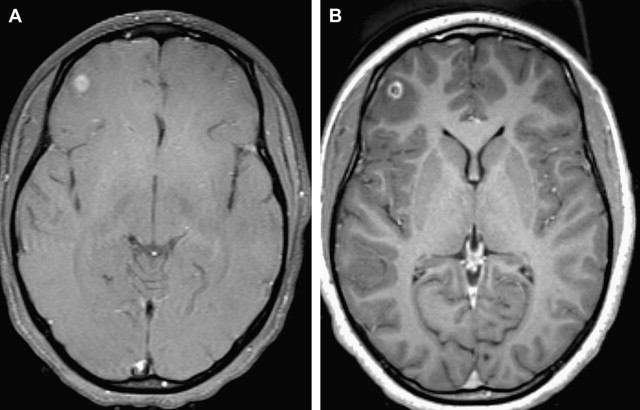
The T1 of many tissues increases in the order of 20% to 50% as the field strength is increased from 1.5 T to 3 T. The T1 of chelated gadolinium (Gd) contrast agents does not increase as much. Because the T1 of the unenhanced tissue typically increases more than the enhancing tissue, equivalent doses of Gd chelate often produce a greater effect at 3 T than at 1.5 T ( Fig. 5 ). This characteristic has been used to reduce the contrast agent dose. The reduced contrast dose has obvious favorable financial and health care implications. Shapiro compared the conspicuity of enhancing lesions on T1 fast spin echo with fast spoiled gradient recalled (FSPGR). Although most lesions were well delineated on both sequences, they varied in conspicuity. Furthermore, some lesions were identified on one but not the other sequence. Our experience is mostly in agreement with these data, although we sometimes note better lesion conspicuity on T1 spin echo sequences compared with the higher-resolution T1 MPRAGE sequences, even considering differences in timing between contrast injection and image acquisition ( Fig. 6 ). However, the reformation of thin slices while maintaining good image quality made possible by 3D volumetric acquisitions may allow better identification of the internal architecture of enhancing lesions on MPRAGE aiding in diagnosis ( Fig. 7 ). Similar to Shapiro, at time of writing, we tend to acquire both sequences after contrast administration, which results in lengthening of the scanning session.
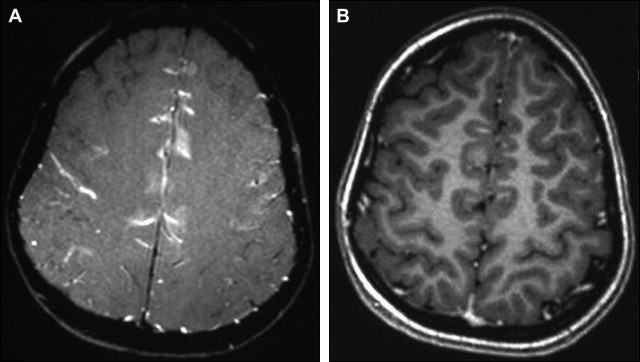
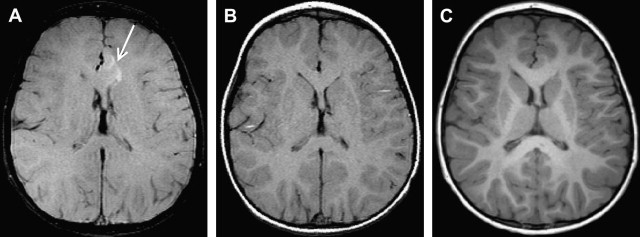
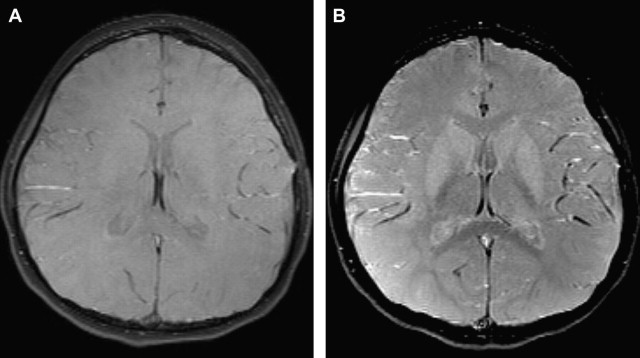
The intrinsic magnetization transfer (MT) effect associated with multislice (with other slices serving as off-resonance excitation) acquisitions can be used in pediatric neuroimaging at higher magnetic field strengths. In our experience, dysplastic lesions such as cortical dysplasia and tubers associated with tuberous sclerosis (TS) often show MT effect, which increases the detectability and allows for more confident diagnosis of focal cortical dysplasia ( Fig. 8 ) and aids in accurate estimation of the lesion burden in TS ( Fig. 9 ). However, if a discrete MT pulse is inadvertently applied to the T1-weighted sequences, many normal structures such as the basal ganglia and parts of the cortex may seem to have abnormal high signal, which could lead to false diagnoses ( Fig. 10 ).
Diffusion imaging
The improved SNR at 3 T makes the use of higher b-values, thinner slices, and (without the need for signal averaging) larger number of diffusion-encoding directions practically possible. This situation results in better quality of diffusion imaging. The inherent sensitivity to susceptibility artifacts in echo-planar imaging (EPI) (which is typically used in diffusion imaging) at 3 T poses a problem by causing image distortion and blurring. These detrimental effects can be partially remedied by the implementation of PI. PI reduces magnetic susceptibility effects and decreases the number of phase encoding steps, shortens the echo train length, and consequently diminishes the effective echo time (TE), making diffusion imaging less sensitive to motion, distortion, and blurring. The result is increased lesion conspicuity and consequently better image quality. Despite the reduction of SNR intrinsic to PI, its use on a 3 T system still generates more SNR than similar sequences performed without PI on 1.5 T magnets. (SNR varies linearly with field and is reduced only by the square root of 2 by using PI with a factor of 2.) In addition, diffusion tensor imaging (DTI) measurements at 3 T are more accurate than 1.5 T, benefiting from reduced distortion and shortened TE (increased SNR, less T2-weighting), DTI quality is improved, and white matter fiber tracking is easier to obtain. This situation helps evaluate the relationship of tumors to the adjacent white matter tracts more accurately, which can play a role in surgical planning. It can also aid in the evaluation of certain brain abnormalities (eg, absence of the decussating fibers of the superior cerebellar peduncles in Joubert syndrome) ( Fig. 11 ).