Chapter Outline
Respiratory Tract, 628
Upper Airway, 628
Congenital Pulmonary Abnormalities, 631
Pneumonia, 634
Neonatal Respiratory Distress, 636
Mediastinum, 638
Gastrointestinal Tract, 639
General, 639
Esophagus, 640
Stomach, 641
Duodenum, Pancreas, SB, 642
Colon, 646
Liver, Biliary Tract, 648
Genitourinary Tract, 649
General, 649
Congenital Anomalies, 650
Renal Cystic Disease, 654
Inflammation, 655
Tumors, 657
Ovarian Masses, 660
Other, 660
Musculoskeletal System, 661
Trauma, 661
Infection, 663
Degenerative and Chronic Traumatic Disease, 664
Metabolic Abnormalities, 669
Congenital Anomalies, 670
Arthritis, 673
Other Disorders, 673
Pediatric Neuroimaging, 674
Differential Diagnosis, 679
Chest, 679
Abdomen, 680
GU System, 683
Central Nervous System, 685
Musculoskeletal System, 686
Other, 690
Respiratory Tract
Upper Airway
Approach
Inspiratory stridor is the most common indication for radiographic upper airway evaluation. The main role of imaging is to identify conditions that need to be treated emergently and/or surgically (e.g., epiglottitis, foreign bodies). Technique:
- 1.
Physician capable of emergency airway intervention should accompany child
- 2.
Obtain 3 radiographs:
- •
Lateral neck: full inspiration, neck extended ( Fig. 11.1 )

FIG. 11.1
- •
Anteroposterior (AP) and lateral chest: full inspiration, include upper airway ( Fig. 11.2 )

FIG. 11.2
- •
- 3.
Fluoroscope the neck if radiographs are suboptimal or equivocal.
- 4.
Primary diagnostic considerations:
- •
Infection (epiglottitis, croup, abscess)
- •
Foreign body (airway or pharyngoesophageal)
- •
Masses (lymphadenopathy neoplasms)
- •
Congenital abnormalities (webs, malacia)
- •
- 5.
If upper airway is normal, consider:
- •
Pulmonary causes (foreign body, bronchiolitis)
- •
Mediastinal causes (vascular rings, slings)
- •
Congenital heart disease (CHD)
- •
- 1.
Normal Appearance
- •
Three anatomic regions:
Supraglottic region
Glottic region: ventricle and true cords
Subglottic region
- •
Epiglottis and aryepiglottic folds are thin structures.
- •
Glottic shoulders are seen on AP view.
- •
Adenoids are visible at 3–6 months after birth.
- •
Normal retropharyngeal soft tissue thickness (C1–C4) = three-quarters of vertebral body width
- •
Laryngomalacia
Common cause of stridor in the first year of life. Immature laryngeal cartilage leads to supraglottic collapse during inspiration. Stridor improves with activity and is relieved by prone positioning or neck extension. Self-limited course. Diagnosis is established by fluoroscopy (laryngeal collapse with inspiration).
Tracheomalacia
Collapse of trachea with expiration. May be focal or diffuse; focal type is usually secondary to congenital anomalies that impress on the trachea, such as a vascular ring.
Webs
Most common in larynx.
Tracheal Stenosis ( Fig. 11.3 )
- •
Diffuse hypoplasia, 30%
- •
Focal ring like stenosis, 50%
- •
Funnel-like stenosis, 20%
- •

Subglottic Stenosis
Fixed narrowing at level of cricoid. Failure of laryngeal recanalization in utero.
Epiglottitis
Life-threatening bacterial infection of the upper airway. Most commonly caused by Haemophilus influenzae . Age: 3–6 years (older age group than with croup). Treatment is with prophylactic intubation for 24–48 hours and antibiotics.
Clinical Findings
- •
Fever
- •
Dysphagia
- •
Drooling
- •
Sore throat
- •
Radiographic Features ( Figs. 11.4 – 11.5 )
- •
Thickened aryepiglottic folds (hallmark)
- •
Key radiographic view: lateral neck
- •
Thickened epiglottis
- •
Subglottic narrowing because of edema, 25%: indistinguishable from croup on AP view
- •
Distention of hypopharynx
- •


Pearls
Other causes of enlarged epiglottis or aryepiglottic folds:
- •
Caustic ingestion
- •
Hereditary angioneurotic edema
- •
Omega-shaped epiglottis (normal variant with normal aryepiglottic folds)
- •
Stevens-Johnson syndrome
- •
Croup
Subglottic laryngotracheobronchitis. Most commonly caused by parainfluenza virus. Age: 6 months to 3 years (younger age group than epiglottitis).
Clinical Findings
- •
Barking cough
- •
Upper respiratory tract infection
- •
Self-limited
- •
Radiographic Features ( Fig. 11.6 )
- •
Subglottic narrowing (inverted “V” or “steeple sign”)
- •
Key view: AP view
- •
Lateral view should be obtained to exclude epiglottitis.
- •
Steeple sign: loss of subglottic shoulders
- •

Pearls
- •
Membranous croup: uncommon infection of bacterial origin (Staphylococcus aureus). Purulent membranes in subglottic trachea.
- •
Epiglottitis may mimic croup on AP view.
- •
Retropharyngeal Abscess
Typically caused by extension of a suppurative bacterial lymphadenitis, most commonly S. aureus , group B streptococci, oral flora. Age: <1 year. Other causes include foreign body perforation and trauma.
Clinical Findings
- •
Fever
- •
Stiff neck
- •
Dysphagia
- •
Stridor (uncommon)
- •
Most cases present as cellulitis rather than true abscess.
- •
Radiographic Features
- •
Widened retropharyngeal space (most common finding)
- •
Air in soft tissues is specific for abscess.
- •
Straightened cervical lordosis
- •
Computed tomography (CT) is helpful to define superior and inferior mediastinal extent.
- •
Plain radiograph findings are usually nonspecific.
- •
Main differential diagnosis (DDx):
Retropharyngeal hematoma
Neoplasm (i.e., rhabdomyosarcoma)
Lymphadenopathy
- •
Tonsillar Hypertrophy
The tonsils consist of lymphoid tissue that encircles the pharynx. Three groups: pharyngeal tonsil (adenoids), palatine tonsil, and lingual tonsil. Tonsils enlarge secondary to infection and may obstruct nasopharynx and/or eustachian tubes. Rarely, bacterial pharyngitis can lead to a tonsillar abscess (quinsy abscess), which requires drainage. Specific causes include:
- •
Mononucleosis (Epstein-Barr virus [EBV])
- •
Coxsackievirus (herpangina, hand-foot-mouth disease)
- •
Adenovirus (pharyngoconjunctival fever)
- •
Measles prodrome (rubeola)
- •
β-Hemolytic Streptococcus (quinsy abscess)
- •
Radiographic Features ( Fig. 11.7 )
- •
Mass in posterior nasopharynx (enlarged adenoids)
- •
Mass near end of uvula (palatine tonsils)
- •
CT is useful to determine the presence of a tonsillar abscess.
- •

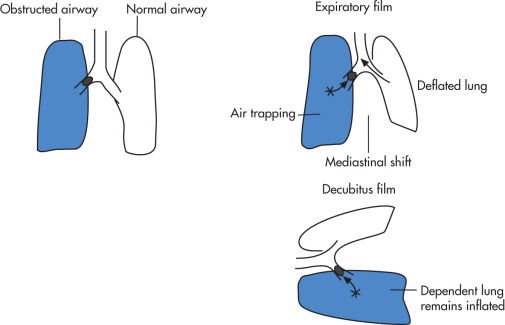
Airway Foreign Body
Common cause of respiratory distress. Age: 6 months to 4 years. Acute aspiration results in cough, stridor, wheezing; chronic foreign body causes hemoptysis or recurrent pneumonia. Location: right bronchi > left bronchi > larynx, trachea.
Radiographic Features ( Fig. 11.8 )
Bronchial foreign body
- •
Unilateral air trapping causing hyperlucent lung, 90%
- •
Expiratory radiograph or lateral decubitus makes air trapping more apparent.
- •
Atelectasis is uncommon, 10%
- •
Only 10% of foreign bodies are radiopaque.
- •
Chest fluoroscopy or CT should be performed if plain radiograph findings are equivocal.
- •
Tracheal foreign body
- •
Foreign body usually lodges in sagittal plane.
- •
Chest radiograph (CXR) is usually normal.
- •
Congenital Pulmonary Abnormalities
Bronchopulmonary Foregut Malformation (BFM)
Arises from a supernumerary lung bud that develops below the normal lung bud. Location and communication with gastrointestinal (GI) tract depend on when in embryonic life the bud develops. Most malformations present clinically when they become infected (communication with GI tract).
| Malformation | Location |
|---|---|
| Sequestration | |
| Intralobar | 60% basilar, left |
| Extralobar | 80% left or below diaphragm |
| Bronchogenic cyst | Mediastinum, 85%; lung, 15% |
| CPAM (formerly CCAM) | All lobes |
| Congenital lobar emphysema | LUL, 40%; RML, 35%; RUL, 20% |
Pulmonary Sequestration
Clinical Findings
- •
Recurrent infection
- •
Lung abscess
- •
Bronchiectasis
- •
Hemoptysis during childhood
- •
Pathology
- •
Nonfunctioning pulmonary tissue (nearly always posteromedial segments of lower lobes [LLs])
- •
Systemic arterial supply: anomalous arteries from the aorta (less common branch of the celiac artery)
- •
No connection to bronchial tree
- •
Radiographic Features
- •
Large (>5 cm) mass near diaphragm
- •
Air-fluid levels if infected
- •
Surrounding pulmonary consolidation
- •
Sequestration may communicate with GI tract.
- •
Bronchogenic Cyst
Result from abnormal budding of the tracheobronchial tree. Cysts contain respiratory epithelium. Location:
- •
Mediastinum, 85% (posterior > middle > anterior mediastinum)
- •
Lung, 15%
- •
Radiographic Features ( Fig. 11.9 )
- •
Well-defined round mass in subcarinal/parahilar region
- •
Pulmonary cysts commonly located in medial third of lung
- •
Initially no communication with tracheobronchial tree
- •
Cysts are thin walled.
- •
Cysts can be fluid or air filled.
- •

Congenital Pulmonary Airway Malformation (CPAM)
Formerly called congenital cystic adenomatoid malformation (CCAM), CPAM refers to a proliferation of polypoid glandular lung tissue without normal alveolar differentiation. Respiratory distress occurs during first days of life. After neonatal period, presentations include recurrent infections and pneumothorax.
Types
- •
Type 0: Rare and fatal
- •
Type 1: Most common, large cysts (2–10 cm), unilateral
- •
Type 2: Common, multiple small cysts (0.5–2 cm), associated with other congenital abnormalities
- •
Type 3: Uncommon, large, often solid
- •
Type 4: Uncommon, thin walled cysts, often multifocal, high association with malignancy, specifically pleuropulmonary blastoma
- •
Pearls
- •
Type 1 and type 4 are difficult to differentiate and carry risk of malignancy (especially type 4). Treatment is resection.
- •
Radiographic Features ( Fig. 11.10 )
- •
Multiple cystic pulmonary lesions of variable size
- •
Air-fluid levels in cysts
- •
Variable thickness of cyst wall
- •

Congenital Lobar Emphysema
Progressive overdistention of one or more pulmonary lobes but usually not the entire lung. 10% of patients have CHD (patent ductus arteriosus [PDA] and ventricular septal defect [VSD]).
Causes
Idiopathic, 50%
Obstruction of airway with valve mechanism, 50%
- •
Bronchial cartilage deficiency or immaturity
- •
Mucus
- •
Web, stenosis
- •
Extrinsic compression
- •
Radiographic Features ( Fig. 11.11 )
- •
Hyperlucent lobe (hallmark)
- •
First few days of life: alveolar opacification because there is no clearance of lung fluid through bronchi
- •
May be asymptomatic in neonate but becomes symptomatic later in life
- •
Use CT to differentiate from bronchial obstruction
- •
Distribution
Left upper lobe (LUL), 40%
Right middle lobe (RML), 35%
Right upper lobe (RUL), 20%
Two lobes affected, 5%
- •

Pulmonary Hypoplasia
Types of Pulmonary Underdevelopment
- •
Agenesis: complete absence of one or both lungs (airways, alveoli, and vessels)
- •
Aplasia: absence of lung except for a rudimentary bronchus that ends in a blind pouch
- •
Hypoplasia: decrease in number and size of airways and alveoli; hypoplastic PA
- •
Scimitar Syndrome (Hypogenetic Lung Syndrome, Pulmonary Venolobar Syndrome)
Special form of hypoplastic lung in which the hypoplastic lung is perfused from the aorta and drained by the inferior vena cava (IVC) or portal vein (PV). The anomalous vein has a resemblance to a Turkish scimitar (sword). Associations include:
- •
Accessory diaphragm, diaphragmatic hernia
- •
Bony abnormalities: hemivertebrae, rib notching, rib hypoplasia
- •
CHD: atrial septal defect (ASD), VSD, PDA, tetralogy of Fallot
- •
Radiographic Features ( Fig. 11.12 )
- •
Small lung (most commonly the right lung)
- •
Retrosternal soft tissue density (hypoplastic collapsed lung)
- •
Anomalous vein resembles a scimitar
- •
Systemic arterial supply from aorta
- •
Dextroposition of the heart (shift because of hypoplastic lung)
- •

Congenital Diaphragmatic Hernia (CDH)
Incidence
1 in 2000–3000 births. Mortality rate of isolated hernias is 60% (with postnatal surgery) and higher when other abnormalities are present. Most reliable predictor of postnatal survival is absence of liver herniation. Respiratory distress occurs in neonatal period. Associated abnormalities include:
- •
Pulmonary hypoplasia (common)
- •
Central nervous system (CNS) abnormalities
Neural tube defects (NTDs): spina bifida, encephalocele
Anencephaly
- •
Types
Bochdalek hernia (90% of CDH): posterior
- •
75% are on the left, 25% on right
- •
Right-sided hernias are more difficult to detect because of similar echogenicity of liver and lung.
- •
Contents of hernia: stomach, 60%; colon, 55%; small intestine, 90%; spleen, 45%; liver, 50%; pancreas, 25%; kidney, 20%
- •
Malrotation of herniated bowel is very common.
- •
Morgagni hernia (10% of CDH): anterior
- •
Most occur on right (heart prevents development on the left).
- •
Most common hernia contents: omentum, colon
- •
Accompanying anomalies common
- •
Eventration
- •
Caused by relative absence of muscle in dome of diaphragm
- •
Associated with:
Trisomies 13, 18, congenital cytomegalovirus (CMV), rubella arthrogryposis multiplex, pulmonary hypoplasia
- •
Radiographic Features ( Fig. 11.13 )
- •
Hemidiaphragm not visualized
- •
Multicystic mass in chest
- •
Mass effect
- •

Kartagener Syndrome
Kartagener syndrome (immotile cilia syndrome) is due to the deficiency of the dynein arms of cilia causing immotility of respiratory, auditory, and sperm cilia.
Radiographic Features
- •
Complete thoracic and abdominal situs inversus
- •
Bronchiectasis
- •
Sinus hypoplasia and mucosal thickening
- •
Pneumonia
Childhood pneumonias are commonly caused by:
- •
Mycoplasma, 30% (lower in age group <3 years)
- •
Viral, 65% (higher in age group <3 years)
- •
Bacterial, 5%
- •
Viral Pneumonia
Causes: respiratory syncytial virus (RSV), parainfluenza
Pearls
- •
All types of bronchiolitis and bronchitis cause air trapping (overaeration) with flattening of hemidiaphragms (best seen on lateral radiograph).
- •
RSV, Mycoplasma, and parainfluenza virus are the most common agents that cause radiographic abnormalities (in 10%–30% of infected children).
- •
Any virus may result in any of the five different radiographic patterns.
- •
Bacterial Pneumonia
The following three pathogens are the most common:
- •
Pneumococcus (age 1–3 years)
- •
S. aureus (infancy)
- •
H. influenzae (late infancy)
- •
Radiographic Features
Consolidation
- •
Alveolar exudate
- •
Segmental consolidation
- •
Lobar consolidation
- •
Other findings
- •
Effusions
- •
Pneumatocele
- •
Complications
- •
Pneumothorax
- •
Bronchiectasis (reversible)
- •
Swyer-James syndrome (acquired pulmonary hypoplasia/postinfectious bronchiolitis obliterans), radiographically characterized by small, hyperlucent lungs with diminished vessels (focal emphysema)
- •
Round Pneumonia
- •
Usually age <8 years
- •
Pneumococcal pneumonia in early consolidative phase
- •
Pneumonia appears round because of poorly developed collateral pathways (pores of Kohn and channels of Lambert).
- •
With time, the initially round pneumonia develops into a more typical consolidation.
- •
Recurrent Infections
- •
Cystic fibrosis (CF)
- •
Recurrent aspirations
- •
Rare causes of recurrent infection:
Hypogammaglobulinemia (Bruton disease; DDx clue: no adenoids or hilar lymph nodes)
Hyperimmunoglobulinemia E (Buckley syndrome)
Immotile cilia syndrome (Kartagener syndrome)
Other immunodeficiencies
BFM
- •
Aspiration Pneumonia ( Fig. 11.14 )
Aspiration pneumonia results from inhalation of swallowed materials or gastric content. Gastric acid damages capillaries causing acute pulmonary edema. Secondary infection or acute respiratory distress syndrome (ARDS) may ensue.

Causes
Aspiration caused by swallowing dysfunction (most common cause)
- •
Anoxic birth injury (common)
- •
Coma, anesthesia
- •
Aspiration caused by obstruction
- •
Esophageal atresia (EA) or stenosis
- •
Esophageal obstruction
- •
Gastroesophageal (GE) reflux, hiatal hernia
- •
Gastric or duodenal obstruction
- •
Fistula
- •
Tracheoesophageal fistula (TEF)
- •
Radiographic Features
- •
Recurrent pneumonias; distribution: aspiration in supine position: upper lobes (ULs), superior segments of LL; aspiration in upright position: both LLs
- •
Segmental and subsegmental atelectasis
- •
Interstitial fibrosis
- •
Inflammatory thickening of bronchial walls
- •
Sickle Cell Anemia
Pulmonary manifestations (pneumonia, acute chest syndrome, and pulmonary fibrosis) are the leading causes of death. Children with acute chest syndrome may present with one or multiple foci of consolidation, fever, chest pain, or cough. Causes: infection (higher incidence), fat emboli originating from infracting bone, pulmonary thrombosis.
Radiographic Findings
- •
Consolidation
- •
Pleural effusion
- •
Fine reticular opacities (pulmonary fibrosis)
- •
Large heart in severe anemia
- •
H-shaped vertebral bodies
- •
Osteonecrosis, bone infarct in visualized humeri
- •
Neonatal Respiratory Distress
Respiratory distress in the newborn is usually due to one of four disease entities:
- •
Respiratory distress syndrome (hyaline membrane disease [HMD])
- •
Transient tachypnea of the newborn (TTN)
- •
Meconium aspiration
- •
Neonatal pneumonia (NP)
- •
| Disease | Lung Volume | Opacities | Time Course | Complication |
|---|---|---|---|---|
| RDS | Low | Granular | 4–6 days | PIE, BPD, PDA |
| Transient tachypnea | High or normal | Linear, streaky a | <48 hours | None |
| Meconium aspiration | Hyperinflation | Coarse, patchy | At birth | PFC, ECMO |
| Neonatal pneumonia | Anything | Granular | Variable |
Respiratory Distress Syndrome (RDS) ( Fig. 11.15 )
RDS (formerly known as HMD) is caused by surfactant deficiency in premature infants. Surfactant diminishes surface tension of expanding alveoli. As a result, acinar atelectasis and interstitial edema occur. Hyaline membranes are formed by proteinaceous exudate. Symptoms occur within 2 hours of life. The incidence of RDS depends on the gestational age (GA) at birth.
| Birth at Gestational Age (wk) | Incidence (%) |
|---|---|
| 27 | 50 |
| 31 | 16 |
| 34 | 5 |
| 36 | 1 |

Radiographic Features ( Fig. 11.16 )
- •
Any opacity in a premature infant should be regarded as RDS until proven otherwise.
- •
Lungs are opaque (ground-glass) or reticulogranular (hallmark).
- •
Hypoaeration (atelectasis) leads to low lung volumes: bell-shaped thorax (if not intubated).
- •
Bronchograms are often present.
- •
Absence of consolidation or pleural effusions
- •
In contrast to other causes of RDS in neonates, pleural effusions are uncommon.
- •
Treatment with surfactant may result in asymmetric improvement.
- •
CXR signs of premature infants:
No subcutaneous fat
No humeral ossification center
Endotracheal tube present
- •

In most cases of RDS, the diagnosis is made clinically but may initially be made radiographically. The role of the radiologist is to assess serial CXRs.
Complications of RDS:
- •
Persistent PDA (signs of congestive heart failure [CHF]); the ductus usually closes within 1–2 days after birth in response to the high P o 2 content.
- •
Barotrauma from treatment: pneumothorax, pneumomediastinum, PIE, and acquired lobar emphysema
- •
Diffuse opacities (whiteout) may be due to a variety of causes:
Atelectasis
Progression of RDS
Aspiration
Pulmonary hemorrhage
CHF
Superimposed pneumonia
- •
Pulmonary Interstitial Emphysema
PIE refers to an accumulation of interstitial air in peribronchial and perivascular spaces. Most common cause: positive-pressure ventilation. Complications:
- •
Pneumothorax
- •
Pneumomediastinum
- •
Pneumopericardium
- •
Radiographic Features
- •
Tortuous linear lucencies radiate outward from the hilar regions.
- •
The lucencies extend all the way to the periphery of the lung.
- •
Lucencies do not change with respiration.
- •
Bronchopulmonary Dysplasia
Caused by oxygen toxicity and barotrauma of respiratory therapy. BPD is uncommon in larger and more mature infants (GA >30 weeks or weighing >1200 g at birth).
| Diagnostic Criterion | Gestational Age Less than 32 wk | Gestational Age Greater than 32 wk |
|---|---|---|
| Time point of assessment | 36 wk PMA a or discharge to home, whichever comes first; treatment with >21% oxygen for at least 28 days plus | >28 days but <56 days postnatal age or discharge to home, whichever comes first; treatment with >21% oxygen for at least 28 days plus |
| Mild BPD | Breathing room air at 36 wk PMA b or discharge, whichever comes first | Breathing room air by 56 days postnatal age or discharge, whichever comes first |
| Moderate BPD | Need a for <30% oxygen at 36 wk PMA or discharge, whichever comes first | Need a for <30% oxygen at 56 days postnatal age or discharge, whichever comes first |
| Severe BPD | Need a for ≥30% oxygen and/or positive pressure (PPV a or nasal CPAP) at 36 wk PMA or discharge, whichever comes first | Need a for ≥30% oxygen and/or positive pressure (PPV or nasal CPAP) at 56 days postnatal age or discharge, whichever comes first |
a Using a physiologic test (pulse oximetry saturation range) to confirm the oxygen requirement.
Complications of BPD include asthma-like disease, pulmonary artery hypertension (HTN) (with or without cor pulmonale), sepsis, and neurodevelopmental impairment.
Meconium Aspiration Syndrome
Meconium (mucus, epithelial cells, bile, debris) is the first stool that is evacuated within 12 hours after delivery. In fetal distress, evacuation may occur into the amniotic fluid (up to 10% of deliveries). However, in only 1% does this aspiration cause respiratory symptoms. Only meconium aspirated to below the vocal cords is clinically significant. Meconium aspiration sometimes clears in 3–5 days. CXR nearly always returns to normal by 1 year of age. History is key for distinguishing between other forms of neonatal respiratory distress.
Radiographic Features
- •
Patchy, bilateral opacities, may be “rope-like”
- •
Atelectasis
- •
Hyperinflated lungs
- •
Pneumothorax, pneumomediastinum, 25%
- •
Complication
- •
Mortality (25%) from persistent fetal circulation
- •
Neonatal Pneumonia (NP)
Pathogenesis
Transplacental infection
- •
Toxoplasmosis, rubella, cytomegalovirus, herpes simplex virus (TORCH)
- •
Pulmonary manifestation of TORCH is usually less severe than other manifestations.
- •
Perineal flora (group B streptococci, enterococci, Escherichia coli )
- •
Ascending infection
- •
Premature rupture of membranes (PROM)
- •
Infection while passing through birth canal
- •
Radiographic Features
- •
Patchy asymmetric opacities in a term infant represent NP until proven otherwise.
- •
Hyperinflation
- •
Transient Tachypnea of the Newborn (TTN)
TTN (wet lung syndrome) is a clinical diagnosis. It is caused by a delayed resorption of intrauterine pulmonary liquids. Normally, pulmonary fluids are cleared by:
- •
Bronchial squeezing during delivery, 30%
- •
Absorption, 30%: lymphatics, capillaries
- •
Suction, 30%
- •
Causes
- •
Cesarean section, premature delivery, maternal sedation (no thoracic squeezing)
- •
Hypoproteinemia, hypervolemia, erythrocythemia
- •
Radiographic Features
Fluid overload (similar appearance to noncardiogenic pulmonary edema)
- •
Prominent vascular markings
- •
Pleural effusion
- •
Fluid in fissure
- •
Alveolar edema
- •
Lungs clear in 24–48 hours.
- •
Extracorporeal Membrane Oxygenation (ECMO)
Mainly utilized in neonates in cases of persistent pulmonary hypertension of the newborn (PPHN), which is an abnormal persistent right-to-left shunt of blood through the fetal circulation, leading to severe hypoxemia.
Etiologies for ECMO use:
- •
Meconium aspiration
- •
Pneumonia
- •
RDS
- •
CDH
- •
Prenatal asphyxia
- •
Idiopathic
- •
- •
<34 weeks GA
- •
>10 days of age
- •
Serious intracranial hemorrhage
- •
Patients who require epinephrine
- •
Complications
- •
Late neurologic sequelae; developmental delay, 50%
- •
Intracranial hemorrhage, 10%
- •
Pneumothorax, pneumomediastinum
- •
Pulmonary hemorrhage (common)
- •
Pleural effusions (common)
- •
Catheter complications
- •
Mediastinum
Thymus
The ratio of thymus to body weight decreases with age. Thymus is routinely identified on CXR from birth to 2 years of age. Size and shape of the thymus are highly variable from person to person.
Common Mediastinal Tumors
Anterior
- •
Thymic hyperplasia and thymic variations in shape and size (most common)
- •
Teratoma
- •
Malignant lymphoma
- •
Cystic hygroma
- •
Thyroid extension
- •
Thymomas are extremely rare.
- •
Middle
- •
Adenopathy (leukemia, lymphoma, tuberculosis [TB])
- •
BFM
- •
Vascular malformations
- •
Posterior
- •
Neuroblastoma
- •
Ganglioneuroma
- •
Neurofibromatosis (NF)
- •
Neurenteric cysts
- •
Meningoceles
- •
Extramedullary hematopoeisis
- •
Pearls
- •
Any pediatric anterior mediastinal mass is considered thymus until proven otherwise.
- •
Posterior mediastinal masses are the most common abnormal chest masses in infants.
Pneumomediastinum
Further detailed in Chapter 1 . A unique radiographic sign in infants is the Spinnaker sign or elevated thymus sign.
Causes
- •
Airway obstruction (foreign body)
- •
Mechanical ventilation
- •
Obstructive lung disease—asthma (Macklin effect)
- •
Trauma
- •
Vomiting (e.g., in patients with bulimia)
- •
Spontaneous
- •
Infection (pneumonia, meconium aspiration)
- •
Gastrointestinal Tract
General
Embryology
The GI tract develops from three embryologic precursors, each of which has a separate vascular supply: foregut, midgut, hindgut.
| Origin | Vascular Supply | Derivatives |
|---|---|---|
| Foregut | Celiac artery | Pharynx, lower respiratory tract, esophagus, stomach, liver, pancreas, biliary tree, duodenum |
| Midgut | SMA | Distal duodenum, small bowel, ascending colon |
| Hindgut | IMA | Transverse colon, rectum, bladder, urethra |
- •
Rotation
- •
Fixation
- •
Canalization
- •
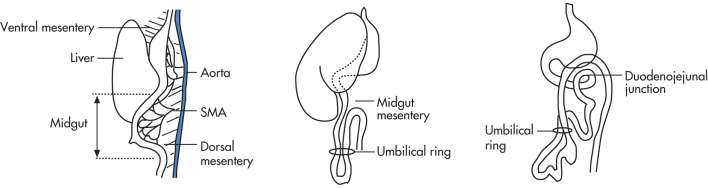
Rotation
- •
Duodenojejunal loop (first 270-degree loop) brings stomach into horizontal axis and puts liver into right and spleen into left abdomen. The common bile duct (CBD) is associated with the ventral pancreas and becomes located dorsally because of the rotation. Before the rotation, the first loop rotation results in the duodenojejunal junction being located to the left.
- •
Cecocolic loop (second 270-degree loop). The small bowel (SB) develops from the dorsal mesentery and then rotates 270 degrees so that the cecum lies near the right upper quadrant (RUQ). It later descends to the right lower quadrant (RLQ).
- •
Fixation
Fixation is incomplete at birth. Abnormal fixation of the colonic mesentery to the posterior abdominal wall results in potential spaces into which the bowel may herniate (paraduodenal hernias). Abnormalities of fixation include:
- •
Increased mobility (cecal bascule)
- •
Internal hernia (paraduodenal, paracecal)
- •
Canalization
After forming a primitive tube, the bowel lumen solidifies and then recanalizes to form the viable lumen. Anomalies in this step may result in atresia or duplication.
- •
Atresia
Duodenal atresia results from failure of recanalization of the foregut (10 weeks) and is frequently associated with other abnormalities (malrotation, 50%; Down syndrome, 30%; EA).
Jejunoileal atresia results from an ischemic insult, not a failure of recanalization; occurs later and is usually an isolated phenomenon.
- •
Duplication
Abnormal recanalization: small intramural duplication cysts may develop.
Notochordal adhesion: a portion of bowel remains adherent to the notochord and a diverticulum may develop.
- •
Umbilical Artery (UA) Line ( Fig. 11.18 )
Traverses the UA, the internal iliac artery, and the infrarenal aorta. Distinguishable from an umbilical venous line because the UA line travels caudally in the UA before it reaches the iliac artery and travels cranially. The tip of the line should be located above the renal arteries between levels T8 and T12. An alternative location of the catheter tip is below the renal arteries between levels L3 and L4.

Umbilical Vein (UV) Line ( Fig. 11.19 )
The UV line should traverse the UV, portal sinus (junction of left and right portal vein), ductus venosus (which closes at 96 hours of life), and IVC and end in the right atrium (RA). The line can easily be misplaced in a portal or hepatic branch (the tip then projects over the liver).

Esophagus
Esophageal Atresia (EA) and Tracheoesophageal Fistula (TEF)
Spectrum of anomalies involving esophagus and trachea. There are two clinical presentations:
- •
Early presentation in EA with a pouch (N-type, 85%): vomiting, choking, difficulty with secretions
- •
Late presentation of recurrent pneumonias if there is a fistula between esophagus and trachea (H-type, 5%)
- •
Types ( Fig. 11.20 )
- •
TEF, N-type fistula, 85%
- •
Pure EA without fistula, 10%
- •
TEF, H-type fistula (no atresia), 1%
- •
Other forms
- •

Associations
VACTERL
- •
V ertebral anomalies
- •
A norectal anomalies
- •
C ardiovascular anomalies
- •
T racheal anomalies
- •
E sophageal fistula
- •
R enal anomalies: renal agenesis
- •
L imb anomalies: radial ray cardiac anomalies
- •
VSD
Ductus arteriosus
Right aortic arch
EA is associated with other atresias and/or stenosis
Duodenal atresia
Imperforate anus
Radiographic Features
Plain radiograph findings
- •
Gas-filled dilated proximal esophageal segment (pouch)
- •
Gasless abdomen (EA-type)
- •
Excessive air in stomach (H-type)
- •
Aspiration pneumonia
- •
Procedures
- •
Pass 8-Fr feeding tube through nose to the level of atresia; distal end of tube marks level of atresia; inject air if necessary
- •
Definite diagnosis is established by injection of 1–2 mL of liquid barium; this, however, should be done only at a large referral institution.
- •
Videotape the injection.
- •
Main DDx
- •
Traumatic perforation of posterior pharynx
- •
Pharyngeal pseudodiverticulum
- •
Ge Reflux
Causes
- •
Immaturity of lower esophageal sphincter during first 3 months of life (usually resolves spontaneously)
- •
Congenital short esophagus
- •
Hiatal hernia
- •
Chalasia (lower esophageal sphincter fails to contract in resting phase; differentiate from achalasia in adults)
- •
Gastric outlet obstruction
- •
Radiographic Features
Barium swallow
- •
Determine presence of reflux
- •
Exclude organic abnormalities
- •
GE scintigraphy
- •
Most sensitive technique to determine reflux and GE emptying
- •
Semiquantitative method
- •
Anatomy only poorly visualized
- •
Esophageal Foreign Body ( Fig. 11.21 )
Foreign bodies in the esophagus may cause airway symptoms by:
- •
Direct mechanical compression of airway
- •
Periesophageal inflammation
- •
Perforation and abscess
- •
Fistulization to trachea
- •


Radiographic Features
- •
Foreign body lodges in coronal plane
- •
Esophagogram may show inflammatory mass.
- •
Fluoroscopic removal with Foley catheter in select instances (e.g., nonembedded)
- •
Stomach
Hypertrophic Pyloric Stenosis (HPS)
Caused by hypertrophy of the circular musculature of the pylorus (differentiate from pylorospasm). Incidence: 1 : 1000 births. Male-female ratio = 4 : 1. Unknown cause. Treatment is with pyloromyotomy.
Clinical Findings
- •
Projectile nonbilious vomiting after each feeding
- •
Peaks at 3–6 weeks after birth
- •
Never occurs after 3 months
- •
Palpable antral mass (olive-sized)
- •
Weight loss
- •
Dehydration
- •
Jaundice
- •
Alkalosis
- •
Associations
- •
EA, TEF, hiatal hernia
- •
Renal abnormalities
- •
Turner syndrome
- •
Trisomy 18
- •
Rubella
- •
Radiographic Features
Plain radiograph ( Fig. 11.22 )
- •
Gastric distention (>7 cm)
- •
Peristaltic waves result in a “caterpillar” appearance to the distended stomach.
- •
Decreased air in distal bowel
- •
Thick antral folds

FIG. 11.22
- •
Ultrasound (US)
- •
HPS appears as target lesion (anechoic mass in RUQ with central echoes because of gas).
- •
Scan in right posterior oblique (RPO) projection (move fluid into antrum)
- •
Size criteria are used to establish the diagnosis of HPS:
Pyloric muscle thickness >3.5–4 mm
Pyloric length >15–18 mm
- •
Useful as first imaging modality
- •
Upper gastrointestinal (UGI) technique ( Fig. 11.23 )
- 1.
Insert an 8-Fr feeding tube to decompress the stomach and drain gastric contents before administration of contrast agent.
- 2.
Use right anterior oblique (RAO) view (to visualize the pylorus)
- 3.
Instill 10–20 mL of barium.
- 4.
Wait for pylorus to open; obtain spot views
- 5.
Get air contrast view by turning patient supine
- 6.
If there is no pyloric stenosis, get lateral and AP views to exclude malrotation.
- 7.
UGI findings of HPS:
- •
Indented gastric antrum (shoulder sign)
- •
Compression of duodenal bulb
- •
Narrow and elongated pylorus: string sign
- •

FIG. 11.23
- 1.
Pylorospasm
Intermittent findings of pyloric stenosis. Treatment is with antispasmodic drugs.
Associations
- •
Adrenogenital syndrome
- •
Dehydration
- •
Sepsis
- •
Radiographic Features
- •
Pyloric musculature is of normal thickness.
- •
Prominent mucosa (echogenic)
- •
Exclude secondary causes of pylorospasm (e.g., ulcer).
- •
Volvulus
Mesenteroaxial volvulus: pylorus lies above GE junction (GEJ).
- •
Occurs with eventration of left diaphragm or diaphragmatic hernia
- •
Acute syndrome: obstruction, ischemia
- •
Organoaxial volvulus: rotation around long axis of stomach
- •
Rare in children
- •
Associated with large hiatal hernia
- •
Lesser curvature is inferior and greater curvature lies superior.
- •
Associated gastric outlet obstruction
- •
Chronic Granulomatous Disease
Inherited genetic disorder (X-linked and autosomal recessive [AR]) leading to dysfunctional nicotinamide adenine dinucleotide phosphate (NADPH) oxidase in phagocytic cells (leukocytes and monocytes), preventing normal respiratory burst. Usually presents at less than 5 years of age.
Clinical Findings
- •
Recurrent bacterial and fungal infections: Recurrent pneumonia (80%), osteomyelitis (30%, especially small bones of the feet and hand)
- •
Lymphadenitis, abscess, and granuloma formation
- •
Hepatosplenomegaly
- •
GI manifestations from granuloma infiltration: esophageal strictures and antral narrowing (characteristic), which may lead to gastric outlet obstruction
- •
Duodenum, Pancreas, SB
Congenital Duodenal Atresia, Stenosis
Results from failure of recanalization (around 10 weeks). Incidence: 1 : 3500 live births. Atresia: stenosis = 2 : 1. Common cause of bowel obstruction. Bilious vomiting occurs within 24 hours after birth. Treatment is with duodenojejunostomy or duodenoduodenostomy.
Associations
- •
30% have Down syndrome.
- •
40% have polyhydramnios and are premature.
- •
Malrotation, EA, biliary atresia, renal anomalies, imperforate anus with or without sacral anomalies, CHD
- •
Radiographic Features
- •
Enlarged duodenal bulb and stomach (double-bubble sign)
- •
Small amount of air in distal SB does not exclude diagnosis of duodenal atresia (hepatopancreatic duct may bifurcate in “Y” shape and insert above and below atresia).
- •
Duodenal Diaphragm ( Fig. 11.24 )
Variant of duodenal stenosis caused by an obstructive duodenal membrane. The pressure gradient through the diaphragm causes the formation of a diverticulum (“wind sock” appearance).

Annular Pancreas
Uncommon congenital ring-like position of pancreas surrounding the second portion of duodenum. Results from abnormal rotation of embryonic pancreatic tissue. The annular pancreas usually causes duodenal narrowing. Diagnosis is made by endoscopic retrograde cholangiopancreatography (ERCP) and magnetic resonance cholangiopancreatography (MRCP).
Pancreatic Tumors
Rare. Well-defined, expansile, less infiltrative compared with adult pancreatic tumors.
Types
- •
Pancreatoblastoma (most common, young children <10 years old): heterogeneous large multilocular septated masses which enhance
- •
Solid pseudopapillary tumor (adolescent girls): low malignant potential, cystic and solid, hemorrhagic, may have calcification.
- •
Islet cell tumors (older children): insulinomas tend to be smaller compared with gastrinomas
- •
Malrotation and Midgut Volvulus
Normally, the rotations place the ligament of Treitz to the left of the spine at the level of the duodenal bulb. Distal end of mesentery is in RLQ. In malrotation the mesenteric attachment is short, allowing bowel to twist around the superior mesenteric vessels ( Fig. 11.25 ). Superior mesenteric vein (SMV) compression (edematous bowel) is followed by superior mesenteric artery (SMA) compression (gangrene).

Associations
- •
Gastroschisis
- •
Omphalocele
- •
Diaphragmatic hernia
- •
Duodenal or jejunal atresia
- •
Radiographic Features
Plain radiograph
- •
Suspect diagnosis in the setting of bowel obstruction and abnormally positioned bowel loops.
- •
A normal position of the cecum does not exclude malrotation but makes it much less likely.
- •
US
- •
Reversal of SMA (right) and SMV (left) location may occasionally be diagnosed by color Doppler imaging.
- •
Distended proximal duodenum
- •
Usually not useful
- •
UGI (gold standard)
- •
Abnormal position of duodenojejunal junction because of absence of ligament of Treitz
- •
Beaking at site of obstruction
- •
Spiraling of SB as it twists around SMA (corkscrew) ( Fig. 11.26 )

FIG. 11.26
- •
Edema of bowel wall
- •
Early diagnosis is essential to prevent bowel necrosis.
- •
Barium enema (BE)
- •
A normal BE will rule out 97% of malrotation.
- •
Can determine if there is a concomitant cecal volvulus
- •
CT
- •
SMA to right of SMV
- •
Gastric outlet obstruction
- •
Spiraling of duodenum and jejunum around SMA axis
- •
Ladd Bands ( Fig. 11.27 )
Peritoneal bands in patients with malrotation. The bands extend from malplaced cecum to porta hepatis and may cause duodenal obstruction.

Ladd Procedure
Treatment for malrotation. Involves counterclockwise derotation of the intestinal twist, widening of the mesenteric root, positioning of the SB on the right hemiabdomen and large bowel on the left, and prophylactic appendectomy.
SB Atresia
As a result of in utero ischemia rather than recanalization failure. Ileum is most commonly affected, followed by jejunum and duodenum; may involve multiple sites. Associated with polyhydramnios in 20%–40%.
Radiographic Features
- •
Dilated SB
- •
Microcolon
- •
Meconium Ileus
In 10% of patients with CF, meconium ileus is the presenting symptom. Thick, tenacious meconium adheres to the SB and causes obstruction, usually at the level of the ileocecal valve. Meconium ileus can be uncomplicated (i.e., no other abnormalities) or complicated (50%) by other abnormalities, such as:
- •
Ileal atresia
- •
Perforation, peritonitis
- •
Stenosis
- •
Volvulus
- •
Radiographic Features ( Fig. 11.28 )
Plain radiograph
- •
Neuhauser sign: “soap bubble” appearance (air mixed with meconium)
- •
SB obstruction
- •
Calcification because of meconium peritonitis, 15%
- •
Enema with water-soluble contrast medium
- •
Microcolon is typical: small unused colon
- •
Distal 10–30 cm of ileum is larger than colon.
- •
Inspissated meconium in terminal ileum
- •
Hyperosmolar contrast may stimulate passage of meconium.
- •

Other Meconium Problems
Meconium Plug Syndrome
Neonatal intestinal obstruction as a result of colonic inertia (in contradistinction to meconium ileus where there is accumulation of meconium in SB). Because of the inertia, the colon usually requires an enema to stimulate peristalsis. Usually occurs in full-term babies and infants of diabetic mothers. In the spectrum with small left colon syndrome. No association with CF.
Meconium Peritonitis
As a result of prenatal perforation of bowel. The inflammatory response may cause peritoneal calcification as early as 12 hours after perforation. Can also observe calcifications in testes.
Meconium Ileus Equivalent
Occurs in older patients with CF, usually patients who do not excrete bile salts. The inspissated fecal material causes intestinal obstruction.
Intussusception
Invagination of a segment of bowel into more distal bowel.
Types
- •
Ileocolic
- •
Ileoileocolic
- •
Ileoileal
- •
Colocolic
- •
Ileocolic and ileoileocolic intussusception make up 90% of all intussusceptions. 90% of all pediatric intussusceptions have no pathologic lead point (hypertrophy of lymphoid tissue); in the remaining 10%, the lead point is due to:
- •
Meckel diverticulum
- •
Polyp or other tumors (lymphoma)
- •
Intramural hematoma
- •
Mesenteric adenitis
- •
Henoch-Schönlein purpura
- •
Clinical Findings
- •
Usually in the first 2 years of life (40% from 3–6 months), rarely in neonates
- •
Colicky crampy pain, 90%
- •
Vomiting, 90%
- •
Sausage-shaped mass, 60%
- •
Blood per rectum (currant jelly stool), 60%
- •
Complications
- •
Bowel obstruction
- •
Intestinal ischemia
- •
Perforation
- •
Sepsis
- •
Dehydration
- •
Radiographic Features
Plain radiograph
- •
Frequently normal (50%)
- •
Intraluminal convex filling defect in partially air-filled bowel loop (commonly at hepatic flexure)
- •
US
- •
Target or doughnut sign
- •
Intussusception Reduction (80%+ Success)
- 1.
Alert pediatric surgeon (in case of perforation).
- 2.
Preliminary kidney, urethra, bladder (KUB) to rule out free air.
- 3.
Insufflate air rapidly, maintaining air pressure below 120 mm Hg. Alternatively, use dilute contrast (e.g., Cysto-Conray 17%) in bag placed 3 feet above table top. Rectal tube may be taped to reduce air leak.
- 4.
Maintain constant hydrostatic pressure but no longer than 3 min against the nonmoving intussusception mass. If patient evacuates around tube, repeat filling may be performed up to 3 tries before surgery is contemplated. (Rule of 3s: bag 3 feet high, 3 min per try, and 3 tries)
- 5.
Successful reduction is marked by free flow of air/contrast into the terminal ileum. Fluoroscopy is used to evaluate for a pathologic lead point. A postevacuation and a 24-hour radiograph are obtained.
- 6.
Contraindications to reduction:
- •
Perforation
- •
Peritonitis
- •
Henoch-Schönlein purpura (predisposes to perforation)
- •
- 7.
Complications
- •
Recurrence in 6%–10% (half within 48 hours after initial reduction)
- •
Perforation during radiographic reduction is rare (incidence: 1 : 300 cases)
- •
- 1.
Henoch-Schönlein Purpura
Small vessel vasculitis presenting with rash, subcutaneous edema, abdominal pain, bloody stools, and arthritis.
Radiographic Features
- •
Intramural hemorrhage of SB
- •
Intussusception, typically ileoileal
- •
Gallbladder (GB) hydrops
- •
Echogenic kidneys
- •
Duplication Cysts
Location: SB (terminal ileum) > esophagus > duodenum > jejunum > stomach (incidence decreases from distal to proximal, skips the stomach). Duplication cysts typically present as an abdominal mass. SB duplications are most commonly located on the mesenteric side; esophageal duplications are commonly located within the lumen.
Radiographic Features
- •
Round fluid-filled mass displacing adjacent bowel
- •
May contain ectopic gastric mucosa (hemorrhage)
- •
Calcifications are rare.
- •
Communicating vertebral anomalies (neurenteric cysts; most common in esophagus)
- •
Omphalomesenteric Duct Anomalies ( Fig. 11.29 )
Omphalomesenteric duct anomalies are due to persistence of the vitelline duct, which connects the yolk sac with the bowel lumen through the umbilicus. Spectrum:
- •
Meckel diverticulum
- •
Patent omphalomesenteric duct (umbilicoileal fistula)
- •
Omphalomesenteric cyst (vitelline cyst)
- •
Omphalomesenteric sinus (umbilical sinus)
- •

Meckel Diverticulum
Persistence of the omphalomesenteric duct at its junction with the ileum. Rule of 2s:
- •
Occurs in 2% of population (most common congenital GI abnormality)
- •
Complications usually occur before 2 years of age
- •
The diverticulum is located within 2 feet of the ileocecal valve.
- •
20% of patients have complications.
Hemorrhage from peptic ulceration when gastric mucosa is present within lesion
Inflammation and ulcer
Obstruction
- •
Radiographic Features
- •
Difficult to detect because diverticula usually do not fill with barium.
- •
Pertechnetate scan is the diagnostic imaging modality of choice to detect ectopic gastric mucosa (sensitivity 95%).
- •
False-positive pertechnetate studies may occur in:
Crohn disease
Appendicitis
Intussusception
Abscess
- •
Colon
Appendicitis
Age: >4 years (most common cause of SB obstruction) (see Chapter 3 ).
Radiographic Features
Plain radiograph
- •
Mass in RLQ
- •
Obliterated properitoneal fat line
- •
Sentinel loop
- •
Fecalith
- •
US
- •
May be useful in children
- •
Thickened appendiceal wall shadowing appendicolith, RLQ abscess
- •
BE (rarely done)
- •
A completely filled appendix excludes the diagnosis of appendicitis.
- •
15% of normal appendices do not fill with contrast.
- •
Signs suggestive of appendicitis:
Beak of barium at base of appendix (mucosal edema)
Irregularity of barium near tip of cecum
Deformity of cecum (abscess, mass effect)
- •
CT
- •
Helical CT with opacification of the GI tract achieved through the oral or rectal administration of 3% diatrizoate meglumine solution is useful to diagnose or exclude appendicitis and to establish an alternative diagnosis.
- •
Necrotizing Enterocolitis (NEC)
Most common GI emergency in premature infants. Precise causes unknown (ischemia? antigens? bacteria?). Develops most often within 2–6 days after birth.
Indication for surgery:
- •
Pneumoperitoneum
- •
Increased incidence in:
- •
Premature infants
- •
Neonates with bowel obstruction (e.g., atresia)
- •
Neonates with CHD
- •
Radiographic Features ( Fig. 11.30 )
- •
SB dilatation: adynamic ileus (first finding), unchanging configuration over serial radiographs
- •
Pneumatosis intestinalis, 80% (second most common sign)
- •
Gas in portal vein may be seen transiently (US more sensitive than plain radiograph); this finding does not imply as poor an outcome as it does in adults.
- •
Pneumoperitoneum (20%) indicates bowel perforation: football sign (floating air and ascites give the appearance of a large elliptical lucency in supine position).
- •
Barium is contraindicated; use water-soluble contrast if a bowel obstruction or Hirschsprung disease needs to be ruled out.
- •

Complications
Acute
- •
Perforation
- •
Later in life
- •
Bowel stricture (commonly near splenic flexure)
- •
Complications of surgery: short SB syndrome, dumping, malabsorption
- •
Complications of associated diseases common in premature infants:
HMD
Germinal matrix hemorrhage
Periventricular leukomalacia
- •
Hirschsprung Disease
Absence of the myenteric plexus cells (aganglionosis, incomplete craniocaudal migration of embryonic neuroblasts) in distal segment of the colon causes hypertonicity and obstruction. Clinical: 80% (male-female ratio = 6 : 1) present in the first 6 weeks of life with obstruction, intermittent diarrhea, or constipation. Diagnosis is by rectal biopsy. Treatment is with colostomy (Swenson, Duhamel, Soave operations), myomectomy. Associated with Down syndrome.
Complications
- •
Intestinal obstruction (in neonates)
- •
Perforation
- •
Enterocolitis 15%, cause uncertain
- •
Radiographic Features ( Fig. 11.31 )
- •
Bowel gas pattern of distal colonic obstruction on plain radiograph
- •
BE is normal in 30%
- •
Transition zone between normal and stenotic colonic segment
- •
Rectosigmoid ratio is abnormal (<1) because of the narrowed segment.
- •
Sawtooth appearance of colon on contrast enema
- •
Significant barium retention on the 24-hour BE radiograph may be helpful.
- •
Transition zone in rectosigmoid, 80%
- •

Congenital Anorectal Anomalies ( Fig. 11.32 )
Failure of descent and separation of hindgut and genitourinary (GU) system in second trimester.
High (supralevator) malformations
- •
Rectum ends above levator sling
- •
High association with GU (50%) and cardiac anomalies
- •
Low (infralevator) malformations
- •
Rectum ends below levator sling
- •
Low association with GU anomalies (25%)
OVERVIEW
Male
Female
High malformation
Fistula to the urethra or less commonly to the bladder
Rectovaginal fistula, hydrometrocolpos
Low malformation (bowel passes through levator sling)
Anoperineal fistula
Fistula to lower portion of urethra, vagina perineum
- •
Associated anomalies are very common:
- •
Sacral anomalies, 30% (tethered cord)
- •
Currarino triad: anorectal anomaly, sacral bone abnormality, and presacral mass
- •
GU anomalies, 30%
- •
VACTERL
- •
GI anomalies: duodenal atresia, EA
- •

Radiographic Features
Plain radiograph
- •
Low colonic obstruction
- •
Gas bubbles in bladder or vagina
- •
Determine location of the gas-filled rectal pouch to the M-line (line drawn through junction of upper two-thirds and lower one-third of ischia; line indicates level of levator sling).
- •
Upside-down views can be obtained to distend distal rectal pouch.
- •
Magnetic resonance imaging (MRI)
- •
Determine location of rectal pouch with respect to levator and to determine if the malformation is supralevator or infralevator type.
- •
Facilitates the detection of associated spinal anomalies
- •
Liver, Biliary Tract
Biliary Atresia
Unknown cause (congenital, autoimmune with perinatal viral infection, toxins?). More common in East Asians. Most common indication for liver transplantation in children.
Types ( Fig. 11.33 )
- •
Type I: Atresia restricted to CBD
- •
Type II: Atresia including common hepatic duct
- •
Type III: Atresia involves left and right hepatic ducts and porta hepatis, most common (>90%)
- •

Main differential is neonatal hepatitis, which is often idiopathic (known causes include viral hepatitis, CMV, rubella, and other viruses). Neonatal hepatitis is a diagnosis of exclusion after other causes of cholestasis have been ruled out.
Radiographic Features
US
- •
Normal GB in 20%
- •
Failure to detect extrahepatic bile ducts
- •
Hepatic iminodiacetic acid derivative (HIDA) scan
- •
No visualization of bowel at 24 hours
- •
Visualization of GB is not helpful and can be seen in 20%.
- •
Good hepatic visualization within 5 minutes excludes diagnosis.
- •
Preprocedural phenobarbital (5 mg/kg per day × 5 days) improves sensitivity of hepatobiliary scans. The finding of normal (≥1.5 cm) or enlarged (≥3 cm) GB is more supportive of diagnosis of hepatitis.
- •
Main DDx is neonatal hepatitis. Findings of neonatal hepatitis include:
Bowel activity present at 24 hours
Decreased and slow hepatic accumulation of tracer
GB may not be seen
- •
One must exclude the presence of CF in patients thought to have biliary atresia. Inspissated bile in CF can be indistinguishable from biliary atresia by US or nuclear scan.
- •
| Hemangioma | Hemangioendothelioma | |
|---|---|---|
| Age | Older children | <6 months |
| Size | <2 cm | 2–15 cm |
| Location | Right lobe | Both lobes |
| Symptoms | No | Hepatomegaly, CHF |
| Ultrasound | Well-defined hyperechoic lesion | Variable |
| Malignant potential | No | Rare |
Hemangioendothelioma
Most common benign pediatric liver tumor. 85% present at <6 months. Associated cutaneous hemangiomas in 50%.
Complications
- •
CHF because of arteriovenous (AV) shunt, 15%
- •
Intraperitoneal hemorrhage
- •
Disseminated intravascular coagulopathy
- •
Thrombocytopenia (Kasabach-Merritt syndrome)
- •
Radiographic Features
- •
Complex, hypoechoic mass by US
- •
Similar signal intensity/contrast characteristics as adult hemangioma
- •
Mesenchymal Hamartoma
- •
Occurs during first 10 years of life
- •
Large multiloculated cystic lesion
- •
Large cysts >10 cm may be seen.
- •
10% are exophytic.
- •
Hepatoblastoma
Most common primary malignant liver tumor in children. Age: <4 years. Elevated alpha-fetoprotein (AFP).
Associations
- •
Beckwith-Wiedemann syndrome
- •
Trisomy 18
- •
Familial adenomatous polyposis
- •
Radiographic Features
- •
Large hepatic mass
- •
Mixed echogenicity (US)
- •
Calcification, 50%
- •
Metastases: lungs > lymph nodes, brain
- •
Hepatocellular Carcinoma (HCC)
Second most common malignant primary liver tumor in children. Age: >3 years. Usually seen in cirrhotic liver. Elevated AFP.
Associations
- •
CF
- •
Autoimmune hepatitis
- •
Hepatitis C
- •
Glycogen storage disease (von Gierke)
- •
Galactosemia
- •
Tyrosinemia
- •
Biliary atresia
- •
Radiographic Features
- •
Difficult to distinguish from hepatoblastoma other than by clinical picture
- •
Lower incidence of calcification than hepatoblastoma
- •
Hypovolemic Shock
- •
Small hyperdense spleen
- •
Marked enhancement of bowel wall, kidneys, and pancreas
- •
Dilated fluid-filled bowel
- •
Small caliber aorta and IVC
- •
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree