Computed tomography (CT) is the imaging modality of choice for evaluating mediastinal masses detected by radiography or clinical presentation. However, CT results can often be indeterminate. Thoracic magnetic resonance (MR) imaging is a noninvasive way to characterize mediastinal lesions, site of origin, and involvement of adjacent structures by providing higher soft tissue contrast than CT, with superior tissue characterization and higher diagnostic specificity. Thoracic MR imaging of mediastinal masses can increase diagnostic certainty, reduce the number of surgical interventions, and improve clinical decision making. In this review article, current imaging techniques and clinical applications of MR imaging as a problem-solving tool for assessing mediastinal masses in pediatric patients are discussed.
Key points
- •
The use of MR imaging in the evaluation of pediatric mediastinal masses has expanded over time because of the intrinsic advantages of MR imaging in providing better tissue characterization as well as heightened awareness of radiation exposure associated with alternative workup strategies that use CT.
- •
MR imaging allows improved differentiation of cystic from solid content and better detection of microscopic fat, hemorrhage, and fibrous content within pediatric mediastinal lesions.
- •
The MR imaging signal characteristics of thymus change over time to reflect the gradual fatty replacement of the thymus, with greater generalized T1-weighted and fast spin-echo T2-weighted hyperintensity, and diminishing intermediate T1- and T2 signal of soft tissue.
- •
The combination of T1/T2 hyperintensity and suppression on fat-suppressed MR imaging pulse sequences helps to confirm the presence of macroscopic fat in pediatric mediastinal masses.
- •
MR imaging can better assess the extent of posterior mediastinal neurogenic tumors and its invasion into the spinal canal than CT due to its superior tissue contrast, allowing for improved surgical planning and management.
Introduction
MR imaging in the pediatric population can be difficult to obtain because of noise, fear, claustrophobia, and inability to remain motionless. Sedation or general anesthesia is often required to obtain images of diagnostically acceptable quality. Despite these limitations, the use of MR imaging in the evaluation of pediatric mediastinal masses has expanded over time due to the intrinsic advantages of MR imaging in providing better tissue characterization as well as heightened awareness of radiation exposure associated with alternative workup strategies that use computed tomography (CT).
As is true elsewhere in the body, MR imaging allows improved differentiation of cystic from solid content and better detection of microscopic fat, hemorrhage, and fibrous content within lesions. MR imaging also offers superior delineation of lesion vascularity, cellularity, and tissue architecture through dynamic contrast enhancement patterns and extent of restricted diffusion. As a result, MR imaging provides higher diagnostic specificity leading to improved clinical management and often prevention of unnecessary diagnostic procedures. In other cases, MR imaging can aid in pre-operative surgical planning by elucidating extent of disease and localizing correct compartment for resection.
MR imaging techniques
MR imaging evaluation of pediatric mediastinal masses is typically performed on a 1.5T or 3T MR scanner with the patient supine, using a phased array receiver coil. Higher field strength imaging on a 3T MR scanner offers several benefits for pediatric imaging, including improved signal-to-noise ratio and contrast-to-noise ratio, with potential for improved spatial and temporal resolution. Compared with adult imaging, patient motion is a more substantial problem with pediatric MR imaging. Sources of motion include voluntary motion from patients’ muscular movements in the scanner, as well as involuntary motion predominantly due to respiratory motion in children unable to suspend respiration on command. Such problems can be minimized by appropriate use of sedation, intubation, and breathing techniques.
Patient preparation
Patients older than 6 years are usually able to cooperate with MR imaging after an explanation of the procedure and reassurance. Distraction techniques, including the use of MR imaging–compatible music and video players, as well as scanning during off hours to minimize ambient noise and activity, can also be helpful. However, light sedation, which allows patients to continuously maintain a patent airway independently and respond appropriately to physical stimulation and/or verbal commands with a minimal depressed level of consciousness, is still generally necessary for uncooperative infants and young children (≤5 year old).
Sedation
Several sedation medications are currently used for pediatric MR imaging, including chloral hydrate, pentobarbital, propofol, and midazolam. Advantages of sedation in children who cannot tolerate MR imaging while awake include reduction in scan time and improvement in image quality. Generally, the least amount of sedation necessary for patients to tolerate MR imaging is administered, both to minimize post-MR imaging side effects and to facilitate patient induction and emergence from sedation. As always, the proper balance should be maintained between adequate sedation for patient comfort and scan performance, and minimization of potential neurologic and cognitive effects associated with prolonged anesthesia.
Breath-Hold Technique in Pediatric Patients
Breath-hold imaging is superior to respiratory gating for elimination of respiratory motion artifact. Older children (>5 years old) are usually able to follow instructions for breath-hold after a practice session before the MR imaging examination. Careful attention to patient breathing and breath-hold rehearsal is essential because inadvertent imaging during poor breath-holds can result in respiratory motion artifact and motion-degraded images. Breath-hold MR pulse sequences should also be tailored to take 15 seconds or less to maximize image quality for the patient’s effort.
In infants and young children (≤5 years old), a controlled ventilation technique is required to obtain breath-hold after sedation and intubation. In sedated and intubated patients, respiratory rate should be set at 40 breaths per minute, and tidal volumes should be as large as practical.
Intravenous Contrast Administration
Contrast agents used for clinical pediatric MR imaging are gadolinium chelated, extracellular contrast agents that cause T1 shortening within blood vessels and perfused tissues. The typical dose for intravenous administration is 0.1 mmol/kg. Gadolinium-based contrast agents (GBCAs) have been shown to pose a risk of severe toxicity in patients with renal impairment, and cases reported in adults indicate the presence of gadolinium accumulation in the central nervous system of patients requiring multiple GBCA-enhanced scans as well as outside the brain, the health risks of which remain unclear. However, administration of a GBCA can add significant diagnostic value in enhancing the conspicuity of lesions and in the evaluation of pediatric mediastinal masses, and thus the addition of GBCA is generally recommended for better lesion characterization.
MR imaging sequences
Basic MR evaluation of mediastinal masses includes T1-weighted, T2-weighted with breath-holding, and pre-contrast and post-contrast fat-suppressed T1-weighted sequences ( Table 1 ). In- and opposed-phase chemical shift gradient echo imaging provides a rapid means of T1-weighted imaging in a single breath-hold (∼20 seconds) and provides additional information regarding the presence of microscopic or intravoxel fat. Respiratory-triggered, radially acquired (motion-corrected) T2-weighted images are preferred when more chest coverage is needed because they allow the patient to rest and breathe quietly during image acquisition. Additional pulse sequences can be acquired depending on the indication and lesion in question including a double-inversion recovery T1-weighted sequence for cardiac tumors, diffusion-weighted imaging to assess the presence and degree of restricted diffusion, and short tau inversion recovery imaging to evaluate for bone marrow edema. Dynamic contrast-enhanced imaging can also be obtained if enhancement pattern is needed for lesion differentiation.
| Sequences | Optional Sequences |
|---|---|
|
|
Spectrum of imaging findings
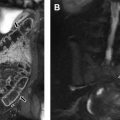
As defined by the International Thymic Malignancy Interest Group (ITMIG) on the basis of CT imaging landmarks, mediastinal masses can be stratified by mediastinal compartment. The ITMIG classification system divides the mediastinum into 3 compartments: the prevascular compartment, the visceral compartment, and the paravertebral compartment ( Fig. 1 ).

The prevascular (anterior) mediastinal compartment contains structures anterior to the pericardium and ascending aorta, including the thymus, fat, lymph nodes, and the left brachiocephalic vein. The visceral (middle) mediastinal compartment is enclosed by the anterior pericardium and extends posteriorly to an imaginary vertical line drawn 1 cm posterior to the anterior cortex of the thoracic vertebral bodies, and includes the trachea, esophagus, heart, and thoracic aorta. The paravertebral (posterior) mediastinal compartment includes all mediastinal structures posterior to this vertical line, and laterally extends to the level of the transverse processes of the vertebral bodies. Table 2 summarizes the differential diagnosis for a mediastinal mass in a child.
| Prevascular (Anaterior) Compartment | Visceral (Middle) Compartment | Paravertebral (Posterior) Compartment |
|---|---|---|
|
|
|
Prevascular (Anterior) Mediastinal Masses
To facilitate diagnosis, prevascular mediastinal masses in children can be classified into one of 3 categories: solid, fatty, and cystic lesions.
Solid lesions
Prominent thymus
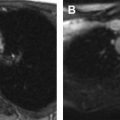
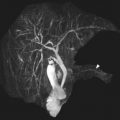
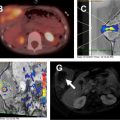
As the normal thymus can take on a variety of shapes and sizes from a quadrilateral shape in infancy and early childhood until the age of about 5 years, to a more triangular shape with straightening of the margins later in childhood, the normal thymus can be misdiagnosed as a mediastinal mass in a child. MR imaging can confirm the presence of a prominent but normal thymus ( Fig. 2 ). The MR imaging signal characteristics change over time to reflect the gradual fatty replacement of the thymus, with greater generalized T1-weighted and fast spin-echo T2-weighted hyperintensity, and diminishing intermediate T1 and T2 signal of soft tissue ( Fig. 3 ). In- and out-of-phase gradient echo imaging can help to differentiate normal thymus from primary thymic neoplasms and lymphoma by demonstrating chemical shift artifact in the normal thymus due to the presence of interspersed fat ( Fig. 4 ). Another important feature to help distinguish normal thymus from neoplastic processes is the lack of mass effect on the adjacent vascular structures or airway.




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree