Neck masses commonly present in children and several potential diagnostic and management pathways exist, though with a paucity of evidence-based recommendations. The purpose of this article is to evaluate the current literature and utilization of various diagnostic imaging modalities , with a review of imaging features and management pearls for pediatric neck masses. A comprehensive understanding and practical imaging workflow will guide optimal patient workup and management.
Key points
- •
Evaluation of neck masses in children requires a practical synthesis of clinical information, laboratory testing, and imaging features.
- •
Major categories of pediatric neck masses include congenital, inflammatory, neoplastic, and vascular, each with distinct imaging features and management pathways.
- •
Diagnostic imaging workup typically begins with ultrasound for superficial lesions or computed tomography for rapid screening, followed by magnetic resonance imaging for detailed soft tissue evaluation.
- •
For potentially surgical lesions, interventional radiology can be consulted for percutaneous biopsy and minimally invasive therapeutic options.
Introduction
Neck masses commonly present in children and several possible diagnostic and management pathways exist, though with a paucity of evidence-based recommendations. The evaluation of neck masses in children requires practical synthesis of clinical information, laboratory testing, and imaging features. Imaging workup typically begins with ultrasound (US) for superficial lesions or computed tomography (CT) for rapid screening, followed by magnetic resonance (MR) for detailed soft tissue evaluation. Nuclear medicine (NM) has selected applications for functional assessment and staging of suspected neoplastic lesions. Following diagnostic imaging, interventional radiology (IR) can be consulted for minimally invasive alternatives to open surgery. Major categories of pediatric neck masses include congenital, inflammatory, neoplastic, and vascular, each with distinct imaging features and management pathways. The purpose of this article is to evaluate the current literature for pediatric neck masses, discuss utilization of various imaging modalities for diagnostic workup, and review characteristic imaging features and management pearls for pediatric neck masses typically encountered in daily clinical practice.
Imaging modalities
Five major imaging modalities for evaluating neck masses in pediatric patients include radiography, US, CT, MR, and NM.
- 1.
US is recommended for initial evaluation of superficial neck masses following physical examination.
- 2.
CT is used for rapid evaluation in acute clinical settings, including suspected infection, trauma, and/or airway compromise.
- 3.
MR provides superior soft tissue evaluation of complex and deep neck masses in the nonurgent setting.
- 4.
NM has selected applications for functional assessment and staging of suspected neoplastic lesions.
- 5.
IR can be consulted for potentially surgical lesions amenable to percutaneous biopsy or minimally invasive therapy.
Radiography
Although radiography can detect sizable neck masses, it has limited utility in characterizing lesion composition, mass effect, and displacement of airway and vascular structures. When a neck mass is suspected to communicate with the airway, dynamic fluoroscopy can be performed during a swallow study or percutaneous contrast injection to evaluate for patent sinus tracts or fistulas.
Ultrasound
US is a useful screening tool for superficial neck masses in children. It is a readily available, inexpensive, and real-time imaging modality that does not involve potentially harmful ionizing radiation. US can characterize important imaging features, including the presence of solid and cystic components, as well as vascularity when Doppler imaging is used. The disadvantages of US include operator dependence with a limited acoustic window and depth penetration. ,
Computed Tomography
CT is a fast and accessible cross-sectional imaging modality that involves ionizing radiation. CT is best used for rapid evaluation of suspected neck masses in acute clinical settings such as inflammation, infection, trauma, hemorrhage, and/or airway compromise. Although CT can distinguish different tissue densities such as calcium, fat, fluid, and blood, it is often limited for complex soft tissue evaluation, even with intravenous contrast administration.
Magnetic Resonance
MR is a more involved examination that uses nonionizing magnetic fields with multiple sequence weightings to enable superior soft tissue discrimination. Because the examination is longer and more detailed, infants and young children who cannot follow breathing instructions may require sedation or anesthesia. Nevertheless, MR imaging represents the current gold standard for evaluating pediatric neck masses, particularly congenital, neoplastic, and vascular lesions with complex anatomic involvement and multiple tissue components.
Nuclear Medicine
NM involves intravenous injection of various radiopharmaceuticals for single photon emission computed tomography (SPECT) and 18-fluorodeoxyglucose for positron emission tomography (PET). NM is often used in oncologic evaluation. SPECT aids in the functional assessment of certain tumors, and PET is useful for whole body staging.
Interventional Radiology
Following diagnostic imaging, IR can be consulted for minimally invasive alternatives to open surgery. Interventional imaging options include angiography for evaluating vascular lesions, image-guided percutaneous tissue biopsy, and minimally invasive therapies such as sclerotherapy and ablation.
Evidence-based imaging algorithm
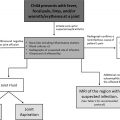
Pediatric neck masses are common and invoke a wide range of diagnostic and management possibilities. Multivariate statistical analysis in large retrospective cohorts has demonstrated that no single clinical or radiologic feature can predict the final diagnosis. Therefore, an ideal workflow for evaluation of pediatric neck masses should appropriately utilize and practically synthesize clinical features, laboratory testing, and imaging findings.
The clinical history should include the age and acuity of onset, recent history of infection or trauma, and any systemic symptoms including fever, night sweats, or weight loss. The physical examination should include location in the neck (medial or lateral, upper or lower, anterior or posterior), presence or absence of pain, mobile or fixed nature, signs of acute inflammation, and any findings outside the neck. Based on clinical assessment, various pertinent laboratory tests may be ordered, such as a complete blood cell count, metabolite concentrations, inflammatory markers, pathogen titers, hormone levels, and tumor markers.
Diagnostic imaging can aid in the detection, localization, and characterization of pediatric neck masses. US is the preferred initial screening study following physical examination for most pediatric neck masses, particularly those that are superficially located. US helps to evaluate lesion location, size, and extent, as well as vascularity on color Doppler imaging. , For deep or complex neck lesions, cross-sectional imaging may be required for complete assessment. CT with iodinated contrast is recommended for rapid evaluation in urgent clinical settings, such as cellulitis, sialadenitis, abscess, and/or airway compromise. CT can also be used to evaluate acute trauma with suspicion for fractures and/or hemorrhage. In nonurgent settings, MR with gadolinium contrast is the current gold standard for evaluating complex soft tissue neck anatomy, including congenital, neoplastic, and vascular lesions. Because the examination is longer and more detailed, affected pediatric patients may require sedation or anesthesia.
Spectrum of pediatric neck masses
Congenital Neck Disorders
Congenital neck anomalies are developmental malformations that are present at birth. Many of these lesions do not come to medical attention until later life, when they present with acute infection or inflammation. Acute inflammatory changes should be treated medically before any planned intervention to decrease the risk of iatrogenic neurovascular injury. The current standard of care involves complete surgical excision to prevent lesion growth, repeat infection, and, in some cases, malignant transformation. Some centers also offer minimally invasive percutaneous sclerotherapy and ablation approaches for benign congenital neck lesions. Four common congenital neck lesions include branchial anomalies, cervical thymus, ectopic thyroid, and germ cell lesions.
Branchial anomalies
The branchial arches are 6 paired embryonic arches that form the structures of the head and neck. Branchial clefts form from ectoderm, branchial arches from mesoderm, and branchial pouches from endoderm. Incomplete obliteration of these embryonic precursors can result in the formation of a cyst, sinus (1 opening), or fistula (2 openings). Imaging helps to evaluate lesion location, size, superinfection, and abnormal communications with surrounding structures.
First branchial anomalies are located between the external ear and mandibular angle. Proposed classifications can be based on morphology (Arnot type I, parotid; II, anterior triangle) or tissue of origin (Work type I, ectoderm; II, ectoderm and mesoderm), but such systems do not correlate well with surgical risk or patient outcomes. , There may be communication with the periauricular soft tissues, parotid gland, parapharyngeal space, or anterior triangle of the neck.
Second branchial anomalies are the most common branchial apparatus anomalies and can be located in the anterior triangle of the neck, posterior to the submandibular gland, lateral to the carotid space, or anterior to the sternocleidomastoid muscle. Historic anatomic classifications exist, but are no longer commonly used in clinical practice (Bailey type I, anterior to sternocleidomastoid muscle; II, abutting internal carotid artery and internal jugular vein; III, between the internal and external carotid arteries; IV, pharyngeal mucosal space). Multiple or bilateral second branchial anomalies can be seen in the setting of brachio-oto-renal syndrome, along with deafness; auricular malformations; preauricular pits or tags; external, middle, and/or inner ear malformations; and renal anomalies.
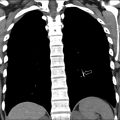
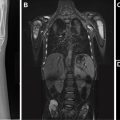
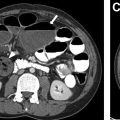
Third branchial anomalies can be located in the upper posterior cervical space or lower anterior neck. Based on embryology, these lesions are often associated with the base of pyriform sinus, above the level of superior laryngeal and hypoglossal nerves and below the glossopharyngeal nerve. Fourth branchial anomalies are typically associated with the apex of the pyriform sinus and present with recurrent abscesses in the lower anterior neck, often in the thyroid gland or mediastinum. The clinical and radiologic features of third and fourth branchial anomalies can overlap greatly, with left lateralization more common owing to developmental asymmetries with persistence of the left thymopharyngeal duct, a remnant of the third pouch; and involution of the right ultimobranchial body, an outpocketing of the fourth pouch ( Fig. 1 ).

Cervical thymus
The thymus is a primary lymphoid organ derived from the third branchial pouch, which descends from the neck into the mediastinum during development. Cervical thymus refers to the presence of ectopic thymic remnants in the neck, which can present as a mass during childhood. Solid remnants can be located in the anterior triangle and deeper fascial layers, sometimes with a residual connection to the mediastinum. Cystic components can be present owing to congenital remnants of the thymopharyngeal duct, or from acquired inflammation or degeneration of thymic tissue. Thymic remnant lesions more commonly occur on the left, again related to persistence of the left thymopharyngeal duct component of the third branchial pouch , ( Fig. 2 ).

Ectopic thyroid
The thyroid is an endocrine gland derived from the fourth branchial pouch. During development, the thyroid anlage migrates from the foramen cecum to the front of the trachea. Therefore, ectopic thyroid tissue can persist anywhere along this paramedian tract from the base of the tongue to mediastinum. Owing to the presence of thyroglobulin, thyroid tissue is inherently hyperdense on CT and T1 hyperintense on MR. It is important to confirm the presence of a normal thyroid gland in the thyroid bed, prior to attempted excision of ectopic thyroid tissue.
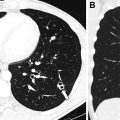
Thyroglossal duct cyst (TGDC) is the most common developmental cyst of the neck and can occur at the suprahyoid, hyoid, or infrahyoid levels. Suprahyoid TGDC is commonly located at the midline base of tongue (foramen cecum) or posterior floor of mouth. Hyoid TGDC is usually located anterior to the midline hyoid bone and may communicate with the airway. Infrahyoid TGDC is often paramedian and embedded in the thyroid strap muscles. Cystic lesions are at risk for malignant transformation to carcinoma, with suspicious imaging features including enhancement, nodularity, and coarse calcifications. Successful treatment involves a Sistrunk procedure with complete resection of the lesion and tract, along with the middle one-third of the hyoid bone ( Fig. 3 ).

Germ cell lesions
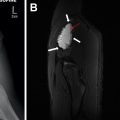
Germ cell lesions are classified based on their embryonic derivatives. The 3 main types are teratoma, dermoid cyst, and epidermoid cyst. Teratomas are true neoplasms containing tissues from all 3 germ cell layers and can be mature (well-differentiated), immature (incompletely differentiated), or malignant (poorly differentiated). The imaging appearance of germ cell lesions is heterogeneous with varying components of soft tissue, calcification, and bulk fat, which can produce substantial mass effect. Dermoid cysts are composed of both ectodermal and mesodermal components, including an epithelial lining and dermal substructure. Internal contents can include hair, macroscopic fat, and calcifications. Epidermoid cysts consist of purely ectodermal components, with a squamous epithelial lining and fluid contents. Both dermoid and epidermoid cysts can present as slowly expanding cystic masses, with recurrent infections when a fistulous tract is present. Internal contents and debris may be visible on US and CT, with restricted diffusion on MR , ( Fig. 4 ).


Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree