Pediatric renal tumors may be malignant or benign. Wilms tumor, the most common malignant pediatric renal tumor, arises sporadically or with various syndromes. Renal cell carcinoma typically presents in older children. Renal clear cell sarcoma and rhabdoid tumor are typically less common, more aggressive, and present in younger children. Benign renal tumors include mesoblastic nephroma, multilocular cystic renal tumor, angiomyolipoma, and metanephric adenoma. Lymphoma and leukemia may secondarily involve the kidney. Although there is overlap in the imaging appearance of several pediatric renal tumors, magnetic resonance characteristics and clinical data narrow the differential diagnosis and suggest a specific diagnosis. This article reviews current MR techniques, as well as the common MR imaging characteristics of malignant and benign pediatric renal neoplasms.
Key points
- •
Wilms tumor is the most common malignant pediatric renal neoplasm.
- •
Several malignant pediatric renal neoplasms may resemble Wilms tumor on MR imaging, although age at presentation and characteristic MR imaging findings may aid in narrowing the differential diagnosis.
- •
MR imaging is a valuable tool in the imaging evaluation of both malignant and benign renal lesions in children.
- •
Certain pediatric benign renal lesions have a characteristic MR imaging appearance, although surgical pathologic assessment may be necessary for definitive diagnosis.
- •
The kidney may be the site of secondary involvement in both pediatric lymphoma and leukemia.
Introduction
MR imaging evaluation of pediatric renal neoplasms provides valuable information that aids in diagnosis and surgical planning. Although Wilms tumor (WT) is overwhelmingly the most common pediatric malignant renal tumor, several additional malignant neoplasms may also be seen in childhood, including renal cell carcinoma (RCC), clear cell sarcoma, and rhabdoid tumor. Furthermore, the kidney may be the site of secondary involvement in both pediatric lymphoma and leukemia. There are multiple benign renal lesions that arise in children, including cystic nephroma, multilocular cystic renal tumor (MCRT), angiomyolipoma (AML), and metanephric adenoma, several of which have a characteristic appearance on MR imaging. The article describes current MR techniques, as well as the common MR imaging characteristics of malignant and benign pediatric renal neoplasms.
Practical imaging approach
At our institutions ultrasound is often the first imaging modality used to evaluate pediatric renal masses. Subsequently, staging is accomplished with computed tomography (CT) or MR imaging. CT remains the most frequently used imaging method due to increased availability, short scanning time that limits or obviates sedation, and simultaneous evaluation of the lungs. MR imaging provides better soft tissue contrast and characterization without ionizing radiation. However, MR imaging has the disadvantages of long scan time and high susceptibility to motion, usually requiring sedation or general anesthesia (GA) in children younger than 7 years of age. CT and MR imaging were found to have a similar diagnostic performance in pediatric renal masses and, as such, either modality may be used for initial staging per recent Children’s Oncology Group (COG) recommendation.
MR Imaging Techniques
Over the last decade, several studies regarding potential long-term neurocognitive deficits as a result of sedation or GA in early life have been published. Although the understanding of these effects is still evolving, reducing the need and duration of sedation for MR imaging is emerging as a priority for pediatric radiologists. Propofol is currently the agent of choice for sedation in MR imaging given its easy titration and fast reversibility, whereas GA is recommended for preterm babies and children at increased risk.
The use of newly developed, accelerated techniques for MR imaging acquisition; for example, parallel imaging, compressed sensing, and multislice acquisition, combined with optimization of MR imaging protocols targeted to answer the clinical question, can lead to significant decrease in scan time and associated GA or sedation. Successful reduction of the need for sedation or GA for MR imaging in children may also be achieved with the help of child life specialists using various distraction techniques, or by “fast and feed” in neonates and infants.
Pediatric patients with renal masses can be scanned in 1.5 or 3 T MR units, using phased array coils. Imaging at 3.0 T provides the advantages of improved resolution due to higher signal-to-noise ratio, increased speed, and potentially better fat suppression, at the cost of increased artifacts and a fourfold increased energy deposition into tissues. A typical MR protocol for renal mass evaluation includes the following sequences: (1) fluid-sensitive sequences that allow characterization of cystic lesions or components; (2) T1-weighted dual-echo (in-phase and opposed-phase) and fat-suppressed images to delineate intracellular and extracellular fat components; (3) diffusion-weighted sequence, which allows identification of areas of high cellularity; and (4) 3-dimensional T1-weighted precontrast and postcontrast images with fat suppression, such as T1-weighted DIXON (Siemens Healthcare, Erlangen, Germany). MR also has the ability of providing multiphase postcontrast imaging that can help improve delineation of the lesions, as well as the evaluation of adjacent vascular structures, without concern for ionizing radiation.
Spectrum of pediatric renal neoplasms
Malignant Pediatric Renal Neoplasms
Wilms tumor
WT is the most common pediatric renal malignancy, representing 5% of pediatric cancers, with an incidence of 7.1 cases per 1 million children younger than 15 years and approximately 650 new cases diagnosed in the United States every year. The vast majority of patients with WT present before the age of 5 years, with a peak incidence around 3 years of age. Although WT is most often sporadic, approximately 10% of cases occur in association with a congenital syndrome or anomaly, such as the combination of WT, aniridia, genitourinary anomalies, and mental retardation (WAGR); Denys-Drash syndrome, characterized by progressive renal disease, male pseudohermaphroditism, and WT; and Beckwith-Wiedeman syndrome, characterized by macroglossia, macrosomia, omphalocele, hemihypertrophy, and WT. Screening renal ultrasound for WT every 3 months from birth (or diagnosis) through the seventh birthday is recommended in all patients with genetic syndromes that have greater than 1% risk of developing WT.
WT is thought to originate from precursors lesions, termed nephrogenic rests, containing clusters of incompletely differentiated metanephric cells, which are normally present in 1% of newborns and usually regress in early childhood. In patients with WT, nephrogenic rests are seen in 35% of cases with unilateral disease and in up to 100% of patients with bilateral renal involvement. Nephroblastomatosis is defined by the presence of diffuse or multifocal nephrogenic rests. Proliferation of these embryonic rests has been associated with mutations in tumor suppressor and transcription genes. The genetic landscape of WT was initially considered limited to a small number of mutations, including WT1, p53, FWT1, and FWT2 genes. Over the last few years, advances in genomic sequencing led to identification of many additional genetic mutations involved in WT oncogenesis.
The prognosis for WT patients has improved dramatically over the last decades, with increased survival from less than 30% to greater than 90%, as a result of cooperative management between international oncology groups leading to improved therapeutic regimens. The 2 most important prognostic factors are tumor histology and stage. Favorable histology implies the absence of anaplasia, whereas unfavorable histology is characterized by the presence of focal or diffuse anaplasia, seen in about 10% of patients, usually older children, and leading to an increased relapse rate and decreased response to chemotherapy, especially for tumors with diffuse anaplasia. Currently, there are 2 staging systems in use. The staging system used in North America and Canada by the COG relies on surgical findings before chemotherapy ( Table 1 ), whereas the staging system used in Europe by the International Society of Pediatric Oncology (SIOP) is based on tumor characteristics at surgery after chemotherapy ( Table 2 ).
| Stage 1 | Tumor confined to the kidney and completely resected with clear margins |
| Stage 2 | Tumor extended in adjacent soft tissues with penetration of the renal capsule or invasion of the renal sinus vessels, still completely resected with clear margins |
| Stage 3 | Incomplete tumor resection with residual nonhematogenous tumor tissue confined to the abdomen |
| Stage 4 | Hematogenous metastatic lesions or lymph node involvement outside the abdominal cavity |
| Stage 5 | Bilateral WTs present at diagnosis |
| Stage 1 | Tumor confined to the kidney or, if extending beyond kidney margins, is surrounded by a fibrous pseudocapsule and completely resected with clear margins |
| Stage 2 | Tumor extends beyond the kidney or fibrous pseudocapsule into renal sinus, blood vessels, lymph nodes, or adjacent organs but is completely resected without evidence of tumor at or beyond the margins of resection |
| Stage 3 | Incomplete tumor resection with residual nonhematogenous tumor tissue confined to the abdomen |
| Stage 4 | Hematogenous metastatic lesions or lymph nodes involvement outside the abdominal cavity |
| Stage 5 | Bilateral WTs present at diagnosis |
WT presents typically as an asymptomatic abdominal mass, discovered incidentally by a parent or relative. Associated signs and symptoms, such as pain, hematuria, or hypertension, are present in only 20% to 30% of the cases. Initial imaging evaluation is usually accomplished with ultrasound, whereas staging is performed with MR imaging and/or CT. WTs are usually large at presentation; however, they tend to displace or invade rather than encase adjacent vessels; this feature is useful in differentiating WT from neuroblastoma. Additionally, calcifications are seen frequently in neuroblastoma, whereas they are present only in approximately 10% of WT. WT can exert significant mass effect on adjacent organs and vessels, which can make the imaging evaluation of these structures challenging.
Given the high soft tissue contrast, MR can provide excellent delineation of the tumor extent and relationship with the adjacent organs, and assessment of vascular involvement and metastatic lesions in lymph nodes, liver, and bone, as well as detection of small synchronous contralateral lesions. MR imaging and CT have a similar high specificity but somewhat low sensitivity when evaluating renal capsular integrity and lymph node metastasis, whereas MR can detect slightly more synchronous lesions than CT.
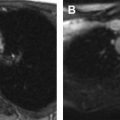
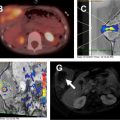
On MR, WT demonstrates T1-weighted isointense to hypointense signal and T2-weighted hyperintense signal, which may be heterogeneous in the presence of hemorrhage or necrosis ( Fig. 1 ). After contrast administration, the lesion tends to show hypoenhancement relative to adjacent renal parenchyma. On diffusion-weighted images, WT typically shows restricted diffusion given increased cellularity. Hales and colleagues recently described a correlation between low apparent diffusion coefficient (ADC) values and high-risk blastemal-type WT, which may allow assessing the risk-based stratification of patients in vivo at an early stage.

Bilateral WT represents 4% to 8% of cases and may present in a synchronous manner, with simultaneous involvement of bilateral kidneys, seen in 6.3% of patients with WT, usually presenting at a younger age than unilateral WT. Metachronous disease implies sequential development of lesions in bilateral kidneys and is present in less than 1% of WT. Therapy in bilateral WT remains challenging given the delicate balance between eradication of tumor and preservation of renal function. Treatment usually entails chemotherapy, attempting to decrease in size the lesions, followed by nephron sparing surgery.
Renal cell carcinoma
RCC represents approximately 4% of pediatric renal cancers, with an increasing rate over the last 20 years. RCC tends to present later than WT in children, in their second decade of life, when RCC is more common than WT. In contradistinction to WT, up to approximately 90% of patients with RCC are symptomatic at diagnosis, most commonly presenting with abdominal pain (43%) or hematuria (37%).
RCC in children has different tumor biology compared with the adult RCC, with translocation RCC with TFE3 gene mutations at Xp11.2 representing the most common form, present in up to 47% of cases. Clear cell carcinoma, which accounts for up to 85% of adult RCC, is rare in children. Clinical course in children is also different from adults, with metastatic nodal disease more frequently encountered. The TNM system is used for staging based on cross-sectional imaging.
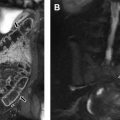
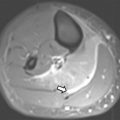
Translocation RCC is associated with prior chemotherapy in approximately 15% of cases and arises from the renal medulla in most affected patients. On MR imaging, translocation RCC is usually hypointense on T2-weighted images and isointense to hyperintense on T1-weighted images, often with a heterogeneous T1-weighted and T2-weighted signal due to cystic and solid components with hemorrhage, necrosis, and calcification ( Fig. 2 ). A fibrotic, low T1-weighted and T2-weighted capsule is often present. On gadolinium-enhanced imaging, the mass demonstrates heterogeneous enhancement, usually less than that of the adjacent renal parenchyma. On diffusion-weighted sequences, RCC may demonstrate hyperintensity.

Adult types of RCC in children include clear cell, often seen in association with von Hippel-Lindau disease; papillary, and chromophobe RCC. Behavior on contrast-enhanced imaging can help in differentiating these subtypes, with clear cell RCC usually demonstrating increased enhancement due to hypervascularity, whereas the chromophobe type has more homogeneous enhancement. Differential diagnosis for RCC includes WT, although WT is typically larger in size at presentation compared with RCC and less frequently contains calcifications. A recent study by Downey and colleagues evaluated imaging features of RCC in 10 pediatric patients found a mean size of 6.2 cm and a maximum dimension of 12.6 cm. Up to 50% of lesions showed internal hemorrhage and/or calcifications, with a heterogeneous appearance on contrast-enhanced cross-sectional imaging present in 90% of cases. All RCC types demonstrate an expansile or ball-shaped growth pattern, which helps differentiate them from tumors with an infiltrative pattern, such as medullary carcinoma, lymphoma, or primitive neuroectodermal tumor (PNET).
Clear cell sarcoma of the kidney
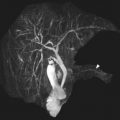
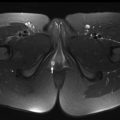
Clear cell sarcoma of the kidney (CCSK) is rare, comprising 2% to 5% of primary pediatric renal tumors. The mean age at presentation is 45 months, with children most frequently presenting between 18 and 54 months of age. There is a male/female ratio of 1.8:1. The typical presenting symptoms include abdominal distention or mass, abdominal pain, and hematuria. 73% of patients present with stage II or greater disease. Lymph nodes are the most common site of metastases at presentation, followed by bone, lung, and liver. On MR imaging, CCSK typically appears as a solid, well-circumscribed, enhancing mass that is T1-weighted hypointense and T2-weighted hyperintense ( Fig. 3 ).