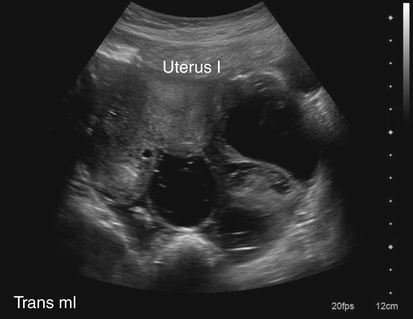
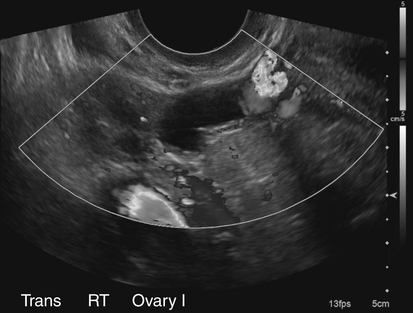
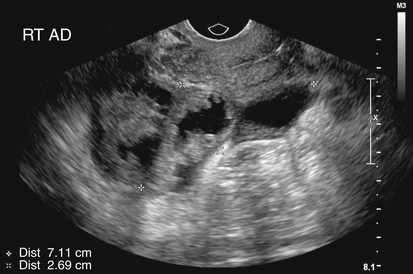
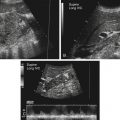
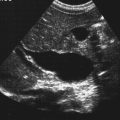
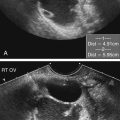
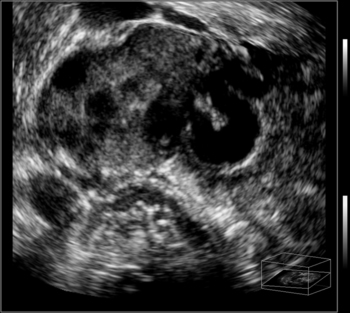
• List and describe the etiology and risk factors associated with pelvic inflammatory disease (PID). • Describe the progression of PID, and identify the specific sequelae of PID. • Describe the sonographic appearances of PID, including endometritis, salpingitis, pyosalpinx, hydrosalpinx, and tuboovarian abscess. • Identify Doppler findings associated with PID. • Identify pertinent laboratory values and related diagnostic studies associated with PID. Pelvic inflammatory disease (PID) is a type of sexually transmitted disease, although bilateral infection may be associated with the use of an intrauterine device (IUD).1 Less commonly, unilateral PID may result from direct extension of primary lower abdominal or pelvic abscesses or complications following abortion or childbirth. The diagnosis of PID is usually made clinically through the assessment of patient history and symptoms, pelvic examination, urine test, and culture of vaginal secretions.2 Early and vigorous antibiotic treatment is needed to stop the progression of the disease and prevent the development of infertility. Normally, the endocervical mucous plug provides a barrier against the ascension of both normal vaginal flora and pathogens. Chlamydia and gonorrhea can damage the endocervical canal and allow an easier ascent of these infectious bacteria into the uterus.3 It also has been noted that other factors may allow for easier spread of disease. Bacteria can gain easier access through the cervical canal shortly after menstruation, when the mucous plug is normally expelled. Easier access also can occur with cervical ectopy, which is the extension of endocervical columnar epithelium beyond the cervix. Cervical ectopy also is more common in teenagers, who represent the age group with the highest incidence of PID.3 The symptoms of acute PID include fever (low or high), shaking chills, abdominal pain (mild, moderate, or severe), nausea, vomiting, vaginal discharge, and irregular vaginal bleeding. Signs of acute PID include abdominal guarding, rebound tenderness, increased pain with cervical or adnexal manipulation, dyspareunia, leukocytosis, elevated ESR, paralytic ileus, and shock from peritonitis.4 Symptoms of chronic PID are persistent pelvic or lower abdominal pain, irregular menses, and possibly infertility. Chronic PID often results in a hydrosalpinx and may include presence of an adnexal mass without fever.3 Peritoneal inclusion cysts also may be seen in women with a history of PID, endometriosis, or prior pelvic surgery or trauma.5 These cysts are created by the trapping of fluid arriving from the ovary by adhesions that are created by an inflammatory process.5 Related diagnostic studies include the following: • Urine test, cultures of vaginal secretions or discharge, culdocentesis, laparoscopy, and computed tomography (CT) and magnetic resonance imaging (MRI) • Endometrial biopsy and histology showing evidence of endometritis—these are the most specific studies for diagnosis of PID2 • Transvaginal sonography or MRI showing thickened, fluid-filled tubes with or without free pelvic or tuboovarian complex or Doppler studies suggesting pelvic infection (tubal hyperemia) Chronic salpingitis is related to recurrent bouts of PID and may result in significant tubal scarring and the presence of hydrosalpinx (Fig. 15-2). The patient may have pain during intercourse or bowel movements (from adhesions involving the bowel and peritoneal surface) and during menses. Tubal scarring may be seen sonographically as several cystic structures extending from the uterus to the adnexa; this is sometimes referred to as the “chain of lakes” or “string of pearls” sonographic appearance (Fig. 15-3). Infertility and ectopic pregnancy may result from the tubal scarring. Pyosalpinx is a progression of PID in which the fallopian tubes become swollen with purulent exudates (Fig. 15-4). The sonographic appearance of pyosalpinx is consistent with visualization of thick-walled tubular or serpiginous structures surrounding the ovaries. The interstitial portion of the tube is tapered at the cornu of the uterus. The tube also may be described as sausage-shaped. Echogenic material or debris related to the presence of pus may be seen within the fallopian tubes. In addition, blurring of the normal tissue planes in the pelvis can occur, making delineation of the organs and structures difficult. Sonographically, the fallopian tubes appear as anechoic thin-walled structures with a multicystic or fusiform mass effect (Fig. 15-5). Color Doppler is useful to differentiate hydrosalpinx from bowel or prominent pelvic veins. The sonographer should take care to show the pathway of the tube and the ovary. Three-dimensional (3D) endovaginal sonography has increased the ability of imaging abnormal fallopian tubes, which may be tortuous and be positioned in a plane that is not easily accessible by standard two-dimensional sonography. In the regular two-dimensional image, multiple cystic areas are seen medial to the right ovary (Fig. 15-6). In the 3D image, a tortuous, fluid-filled tube is easily identified (see Fig. 15-5).
Pelvic Inflammatory Disease
Sonographic Findings
Fallopian Tubes
Salpingitis
Pyosalpinx
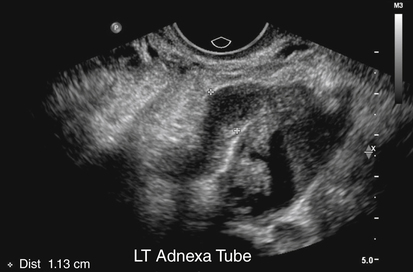
Hydrosalpinx
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Pelvic Inflammatory Disease