Nomenclature
Description of dissection
Historic definition
Zones of dissection
Current MSKCC definition
External iliac vein
Limited
1
Limited
External iliac vein and obturator fossa
Standard
1, 2
Limited
External iliac vein, obturator fossa, and hypogastric vessels
Extended
1, 2, 3
Standard
External iliac vein, obturator fossa, hypogastric vessels, and common iliac to ureteral crossing
Super-extended
1, 2, 3, 4
Extended
Regardless of the definition, the extent of the template that should be used for a PLND remains controversial. Many practitioners perform a limited nodal sampling, as evidenced by the low overall nodal yields recorded in population-based databases such as the Surveillance, Epidemiology, and End Results (SEER) registries. In 2006, according to SEER, the median number of lymph nodes removed during PLND was six, indicating a substantial decline over the previous two decades [13]. While the reasons for these disturbing trends are not altogether clear, it may be that practitioners are unconvinced by data indicating the therapeutic nature of PLND, or there is lack of clarity regarding which nodes to remove, or perhaps because more-extended PLNDs require more time, effort, and skill, with a concurrent increase in the risk of complications associated with the procedure [23]. In an effort to determine the appropriate template for PLND, a few small, randomized trials have compared limited with more extended templates, but because of their small size and single-institution design, they have failed to resolve the issue [24, 25].
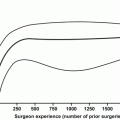
Despite these uncertainties, there are some principles that can provide clarity. First, and perhaps most important, the more nodes removed, the higher the probability of discovering a positive node. Because a positive node can only be detected if it has been resected, a negative PLND will only be truly negative if a positive node has not been inadvertently omitted at the time of resection. PLNDs with small nodal yields have higher false-negative rates than dissections with greater numbers of nodes removed. Briganti et al. [26] eloquently demonstrated this principle by creating receiver operating characteristic (ROC) curves demonstrating that the ability to detect a positive node is dependent on the number of nodes removed. They found that 28 nodes need to be removed and examined to achieve a 90 % probability of detecting a positive node, whereas the probability is less than 10 % when ten or fewer nodes are removed.
Unfortunately, measuring lymph node yield as a surrogate for discerning adequacy of dissection has limitations. There may be significant variation in nodal yield from one patient to the next, even when the same PLND template is performed by the same surgeon and analyzed by the same group of pathologists in the same manner [27]. This variation may reflect differences in the number of nodes in the individual patient or subtle differences in either the collection or processing of the specimen. Lymph nodes smaller than 1 cm are difficult to detect in bulky or en bloc specimens, whereas individually labeled, less bulky packets are easier for pathologists to dissect. Methods to increase nodal yield (which usually also increases the cost of the procedure) may include sending separate nodal packets to pathology rather than sending en bloc specimens [28].
Packeted dissections may provide higher nodal yield, but perhaps more importantly they allow surgeons to attribute nodal metastases to a specific anatomic location. Bader et al. performed packeted dissections on 365 patients, dividing the PLND into external iliac, obturator, and internal iliac packets based on the anatomic boundaries of the dissection [5]. The authors found nodal positivity in 88 patients; positive nodes were located along the internal iliac artery in more than half of these patients (58 %) and exclusively in this location in 19 % of patients. A more recent study by another group of investigators reported similar findings. Of 642 consecutive patients, 35 were found to have nodal metastasis; of them, 80 % had only one (49 %) or two (31 %) positive lymph nodes. Isolated nodal positivity was found more commonly in either the obturator or hypogastric fossa than the external iliac fossa [6]. Most important, the authors demonstrated that a PLND limited to the external iliac area would have identified and removed positive nodes in only a third of the patients actually found to have node positivity on a full PLND. Multiple other studies have confirmed these findings, indicating that positive nodal tissue is often found exclusively below the obturator nerve or along the hypogastric artery [7, 29]. Together these investigators have clearly established that, at minimum, a PLND should include all the tissue from the iliac bifurcation cranially to Cooper’s ligament caudally, from the pelvic sidewall laterally to the urinary bladder medially, and from the external iliac vein superiorly to the pelvic floor inferiorly, exposing the hypogastric vein and skeletonizing the obturator vessels. Minimal templates should include zones 1, 2, and 3 (referred to at MSKCC as a “standard dissection”), but for patients with higher risk tumors these templates are often expanded to include zone 4 or beyond (referred to at MSKCC at an “extended PLND”).
Many have suggested that PLND templates should be expanded to include tissue cephalad to the iliac bifurcation—along the common iliac up to or above the ureteric crossing—or include tissue medial to this region in the presacral area [29–31]. These extended or superextended templates have been advocated by some investigators, either because of lymphoscintigraphy studies demonstrating they capture a greater proportion of the prostate’s primary landing zone or because isolated positive nodes have been identified in these regions. Templates above the aortic bifurcation have also been attempted but have revealed that nodal positivity is only found above this region when multiple nodes (five or greater) are positive within the pelvis [32]. Because lymph node dissection only provides durable therapeutic benefit when limited numbers of nodes are positive, the benefit of a PLND extending above the aortic bifurcation must be questioned until there is clear evidence to the contrary [9, 10]. (See “Therapeutic Benefit of PLND,” below.)
Can Adequate PLND be Performed Using a Robotic Approach?
The surgical technique of RP has changed dramatically since 2001, when the da Vinci surgical platform was first introduced. Currently there are more than 2,500 robotic systems installed worldwide [33]. In the USA, robot-assisted laparoscopic RP was rapidly adopted and is now the most commonly used technique for performing RP [34]. Unfortunately, population-based studies have demonstrated that the probability of receiving a PLND at the time of RP is lower when the surgery is performed using the robotic technique [11]. The exact cause for this disparity is not completely clear but likely stems from (1) the lack of formal robotic and/or oncologic training by most early adopters of robotic techniques, and (2) concern regarding the potential difficulty in addressing major vascular complications with robotic systems.
Many of the largest published series establishing the robotic approach as a viable alternative to open RP have included little to no information regarding PLND [35]. Consequently, questions remain regarding the proportion of patients who receive a concurrent PLND, the extent of the templates used, or typical lymph node yields or density. Several published reports comparing outcomes after open and robotic approaches for RP and/or PLND have failed to show that an equivalent PLND dissection can be performed robotically; and each of these studies has been limited in various ways (Table 4.2). However, recent studies have begun to establish that when attention and focus is placed on R-PLND by experienced surgeons, an equivalent number of positive nodes can be obtained and equivalent oncologic outcomes can be achieved [36]. Nodal yields, like biochemical recurrence and margin status, ultimately depend more on the surgeon than on the surgical approach [36–38].
Table 4.2
Comparative studies of lymph node yields for open and robotic pelvic lymph node dissection during radical prostatectomy
Nodal yield | ||||||
|---|---|---|---|---|---|---|
Study | Institution | Number of patients | Open | RALP | P value | Limitations |
Zorn et al. [39] | University of Chicago Medical Center | 767 | Mean 15.0 | 12.5 | <0.01 | Contemporary RALP series was compared to historical open series |
Cooperberg et al. [40] | University of California, San Francisco, Cancer Center | 524 | Mean 14.4 | 9.3 | <0.01 | Only a small fraction of patients received PLND |
Truesdale et al. [41] | Columbia University Medical Center | 316 | Mean 7.5 | 6.3 | 0.06 | Open group was at greater risk of lymph node involvement |
Silberstein et al. [36] | Memorial Sloan-Kettering Cancer Center | 323 | Median 20.0 | 16.0 | 0.01 | Differences between surgeons were greater than differences between techniques |
Complications
Complications directly attributable to PLND during RP are rare, but the true number is difficult to ascertain because undergoing a simultaneous RP is more likely to cause complications [42, 43]. The most common complication resulting from PLND is leakage of the lymphatic fluid into the space surrounding the transected lymphatic channels. Ideally, at the time of PLND all open lymphatic channels will be ligated and sealed, but there is a risk of some open channels remaining and subsequent leakage. This lymphatic fluid may leak into the peri-lymphatic space and, rarely, cause problems. Symptoms depend on the size, location, and potential for this fluid collection to get secondarily infected. Symptomatic lymphoceles can result in abdominal or pelvic distention, pain, deep venous thrombosis by compressing on the iliac veins and disrupting laminar blood flow, potential disruption of the urethral anastomosis, or superinfection. Because lymphoceles are detected only when they are symptomatic, their true incidence is underestimated but has been reported to be as high as 26–51 % when routine imaging is performed [44, 45]. The rate of symptomatic lymphoceles is widely variable and reported to range from 5–15 % [45, 46]. The wide variation likely reflects differences in surgical technique, patient characteristics, and detection methods. While surgical technique is the most prominent causative factor of lymphocele, other influencing factors include the extent of dissection [46], presence of lymph node invasion, use of heparin [47], use of cautery, or prior radiation. Various strategies have been suggested for mitigating lymphocele formation, including use of preoperative heparin in an upper rather than lower extremity [48] or use of fibrin sealants to the operative bed [49, 50]. Meticulous surgical technique that includes careful dissection and generous use of clips to ligate all transected lymphatic channels likely has the greatest impact in preventing this complication.
While surgical technique may be critical in the prevention of lymphocele formation, surgical approach may not be. The hypothesis that transperitoneal laparoscopic approaches may be associated with lower rates of lymphocele formation due to the ability of the lymphatic fluid to drain into the peritoneum for reabsorption may be unfounded. In one study, routine CT following PLND in 76 patients noted lymphoceles in 51 % of patients, with 15 % being clinically symptomatic [45].
When a lymphocele does become symptomatic and treatment is required, percutaneous drainage is the most common technique utilized. Once a drain is placed, the fluid is aspirated and tested for creatinine to rule out a urine leak, and bacterial cultures are obtained to direct appropriate antibiotic therapy. Simple drainage may not resolve the issue alone, and continued lymphatic drainage may require injection of a sclerosing solution to seal the open lymphatic. Multiple sclerosing agents have been used, including tetracycline, doxycycline, bleomycin, ethanol, povidone iodine, and sodium amidotrizoate [51]. Alternatively, the lymphatic collection maybe treated with open drainage or marsupialized within the peritoneal cavity. Both practices are reported to have greater success rates than percutaneous drainage, but with greater levels of intervention and higher rates of morbidity [52].
Most patients undergoing RP and PLND are considered to be at high risk for the development of thromboembolic events by virtue of their age (typically >50 years), known malignancy, and pelvic surgery [53]. However, because thromboembolic events are rare and may go undetected, determining the impact that PLND has on potentiating this risk is challenging [39, 53]. Lymphocele formation has been demonstrated to be associated with greater risk of deep venous thrombosis formation, again highlighting the need for meticulous ligation of all lymphatic channels [46].
Though rare, injuries to nerves within the pelvis, most notably the obturator nerve, can occur during PLND. The obturator nerve enters the pelvis behind the iliac vessels, runs laterally along the pelvic sidewall, and exits through the obturator foramen. The nerve provides motor innervation of the adductor muscles of the thigh and sensory innervation to the skin on the medial aspect of the proximal thigh. The fibrofatty tissue removed during PLND completely surrounds the nerve, obscuring its visualization at the start of the dissection. This tissue needs to be carefully dissected off the nerve, and during this process the nerve may be subject to partial or complete transection, or stretch, thermal, or crush injury. If transected, the nerve may be approximated with fine non-absorbable suture.
During PLND, particularly when an extended template dissection is performed with dissection of tissue along the course of the common iliac vessels, there is risk of injury to the ureters as they enter the pelvis. Depending on the extent of the injury, ureteral reimplantation or stenting may be required. A detailed understanding of the pelvic anatomy and the relationship between the various structures is necessary to avoid neural, vascular, or ureteric injury during PLND.
Of the possible intraoperative complications that can occur during a robot-assisted operation, the most challenging and potentially life threatening is sudden hemorrhage that is not controllable with the robotic platform. A major vascular complication during open PLND can usually be controlled by tamponading the bleeding vessel with a sponge stick or a vascular clamp. In the case of a robotic procedure, the situation can be more difficult. Immediate conversion to an open procedure may be necessary to achieve vascular control as quickly as possible. This process is impeded by several factors, including the primary surgeon not being at the bedside, the robot blocking access to the patient, and standard robotic instruments being inadequate for this specific task. When residents or fellows are taught to perform RP in a stepwise fashion, PLND is often the first portion of the procedure taught during open technique, but it is usually the last part taught during robotic training, due to the potentially life-threatening situation caused by an inadvertent vascular injury.
Who Should Receive a PLND?
Omitting a PLND avoids potential complications, decreases the length of surgery, and saves $900–$3,000 in costs associated with the procedure [54]. But omission fails to provide important staging information and potential therapeutic value. Because the incidence of lymph node metastasis in most published surgical series is relatively low (usually below 10 %), various predictive tools such as nomograms have been proposed in order to maximize the benefit and minimize overtreatment. These nomograms have been created to determine the probability of lymph node involvement at the time of RP based on PSA, clinical stage, and Gleason score. Various guidelines suggest different cutoffs (Table 4.3). For example, the National Comprehensive Cancer Network (NCCN) guidelines recommend that PLND be performed in all patients undergoing RP who are estimated to have a ≥2 % risk of lymph node involvement, since this avoids 48 % of PLNDs at a cost of missing 12 % of positive lymph nodes [55]. The European Association of Urology (EAU) guidelines use a 5 % cutoff, in which the estimated risk of nodal involvement is between 15 and 40 % [56]. An important limitation of most nomograms is that they are based on cohorts of patients who underwent limited PLND and may therefore underestimate the true risk of nodal metastasis [57, 58]. A more complete evaluation of all existing nomograms is published elsewhere [15]; however, only two nomograms have been created and validated in sets of patients that have undergone extended PLND [59, 60]. Because both the American Urological Association (AUA) and EAU guidelines recommend extended PLND, these two nomograms likely more accurately predict true rates of nodal metastasis.
Table 4.3
Guidelines for pelvic lymph node dissection
Group | Recommendation | Limitations |
|---|---|---|
AUA [61] | PLND is generally reserved for patients with higher risk of lymph node involvement | Fails to define “higher risk” and does not specify the extent of PLND |
NCCN [55] | Extended PLND is recommended for all patients with a ≥2 % risk of lymph node involvement | Does not specify which nomogram to use to determine risk |
EAU [56] | Extended PLND is recommended for all patients with a ≥5 % risk of lymph node involvement | Does not specify which nomogram to use to determine risk |
MSKCC | PLND that includes external, internal, and obturator lymph nodes is recommended for all patients with a nomogram-estimated ≥2 % risk of lymph node involvement and for some patients with a <2 % risk, at the discretion of the surgeon | Unclear what factors should influence the surgeon’s decision to perform PLND for patients with <2 % risk |
The AUA recommendations are the vaguest of the major guidelines regarding indications for and extent of PLND [61]. These state that PLND is “generally reserved for patients with higher risk of lymph node involvement,” but fail to give specific cutoffs or definitions of “higher risk” disease.
In general, we recommend guiding patients with lower risk cancer toward active surveillance and reserving definitive treatment for patients with higher risk disease. As active surveillance becomes a more common recommendation for men with low-risk prostate cancer, the remaining pool of patients who undergo surgery will likely have more aggressive disease. Consequently, a higher percentage of the patients who receive an RP are likely to also benefit from a PLND [62]. Furthermore, if a PLND is performed, we recommend removal of all fibrofatty tissue surrounding the external, obturator, and internal vessels, from the node of Cloquet to the bifurcation of the internal/external iliac vessels. For patients at particularly high risk or with radiographic suspicion of nodal metastasis beyond these boundaries, the template may be expanded further.
Therapeutic Benefit of PLND
PLND provides staging information that can help to determine the extent of the patient’s disease for both prognostic and treatment planning purposes. Removal of pelvic lymph node tissue—especially in the setting of minimal regional spread—can also provide therapeutic benefit. Multiple studies have demonstrated that a significant portion of patients with limited nodal involvement and favorable histopathologic features will not experience recurrence or progression following surgery, even without adjuvant treatment. Catalona et al. first demonstrated a 75 % recurrence-free survival rate at 6 years for patients who had undergone RP and had node-positive disease [63]. In the PSA era, Bader et al. demonstrated that the number of positive nodes correlated with the risk of biochemical recurrence following RP; for example, 42 % of patients with only one positive node remained free from biochemical recurrence at a median follow-up of 45 months [10]. More recently, Von Bodman et al. performed a retrospective review of 162 patients with lymph node metastases who received no adjuvant treatment. They found that patients with one positive node and a Gleason score of seven or lower had a recurrence-free probability of 79 % 2 years after RP, and those with greater numbers of positive nodes had greater rates of biochemical recurrence [9].
Detecting nodal positivity may direct subsequent adjuvant treatments with resulting therapeutic benefit. For example, adjuvant hormonal therapy in patients with positive lymph nodes may produce a long-term survival benefit compared with observation, as demonstrated by Messing et al. In a prospective randomized trial of 98 patients with prostate cancer, immediate long-term androgen deprivation therapy was associated with improved overall survival advantage, with a hazard ratio of 1.84 (95 % confidence interval 1.01–3.35, p = 0.04) at a median follow-up of 11.9 years [64]. This highly provocative study has received much criticism for its small size, lack of standardized PLND template, and the multiplicity of surgeons and centers involved. Additionally, it was conducted early in the era of widespread PSA screening, and the disease extent (i.e., disease stage at time of initial diagnosis) of these patients may not reflect current practice. Therefore, despite the overall, progression-free, and disease-specific survival advantages demonstrated in this study, we do not advocate adjuvant therapy for all patients. Men with minimal nodal disease found with thorough dissection can be carefully watched, receiving adjuvant treatment only after biochemical or clinical recurrence.
Interestingly, population-based studies have demonstrated that even in the absence of any pathologically positive lymph nodes, PLND may provide therapeutic benefit. Joslyn and Konety reviewed the SEER database and, after controlling for multiple patient- and disease-related factors, noted in a multivariate analysis that, even when restricting analysis to node-negative patients, there was a decreased risk of prostate cancer death when ten or more nodes were removed [65]. This benefit may be due in part to micrometastatic disease that is present in the nodal tissue but falsely determined to be negative on histopathologic observation [66]. Several authors have noted that reverse transcriptase polymerase chain reaction may identify such nodal metastases [67, 68]. While these tools are intriguing, they are currently most commonly reserved for the research setting.
Surgical Technique of Robot-Assisted Pelvic Lymph Node Dissection
The majority of RPs performed in the USA today is done via a robotic approach [34]. A successful robotic PLND requires not only a meticulous dissection but also a high degree of comfort and skill on the part of the surgeon in the use of the robotic platform. One of the fundamental keys to successful surgery, whether done by an open or minimal access approach, is learning how to achieve and maintain the exposure necessary to accomplish the task at hand. This necessitates a detailed knowledge of the anatomy of the region and an understanding of the most efficient use of traction and countertraction to facilitate dissection. Robotic surgery has an enormous, but often neglected, advantage in this regard. The robot allows the operator to become a three-handed surgeon, and with the help of a single assistant, a five-handed one. At any moment the surgeon can engage two of the robotic arms to provide traction and countertraction, while at the same time positioning and directing the bedside assistant to do the same through two assistant ports. Simultaneously the surgeon uses the remaining, or “fifth,” hand for the primary task of cutting and dissecting. Patient position, bedside assistant utilization, and port placement become critical components in this process of efficient and successful robotic surgery.
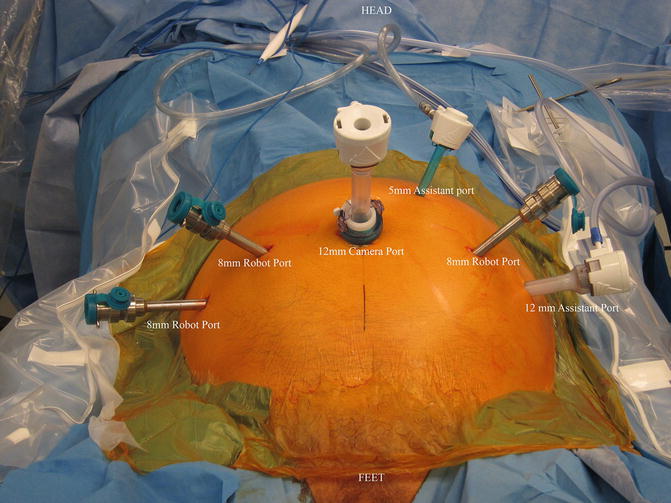
When utilizing an intraperitoneal approach for robotic RP with PLND, port placement is modified only slightly, in that a slightly more cranial port placement is favored. The camera is inserted via a supraumbilical semi-circumferential incision and all additional ports are placed at or above the level of the umbilicus, or higher if the dissection is to be carried up to the inferior mesenteric artery as in a cystectomy. Surgeon preference varies as to the number and position of ports. For a right-handed surgeon, placing two robotic arms on the patient’s right and one on the left is our preferred approach. This setup allows for opposing graspers, one on the left and one on the right, to provide traction and countertraction while keeping the dominant hand free to manipulate the scissors. When set up this way, instrumentation typically includes Maryland or fenestrated bipolar forceps in the left hand and ProGrasp forceps or similar device in the third arm on the far right. Scissors are placed on the right side in the number-one robotic port. Having grasping abilities in opposing hands facilitates achieving and maintaining exposure on either side of the pelvis. With this setup, the assistant will be on the patient’s left side and work through two accessory ports—a 5 mm between the camera and left robotic port, and a 12 mm on the far left. While an accessory 12 mm port is not absolutely necessary, it allows for a reusable specimen bag to be placed multiple times in order to retrieve the nodes in as many packets as the surgeon desires, and then it is available for prostate removal at the end of the procedure. Another advantage to an accessory 12 mm port is that the robotic camera can be placed through this port to look at the umbilical port if midline adhesions are encountered (Fig. 4.1).