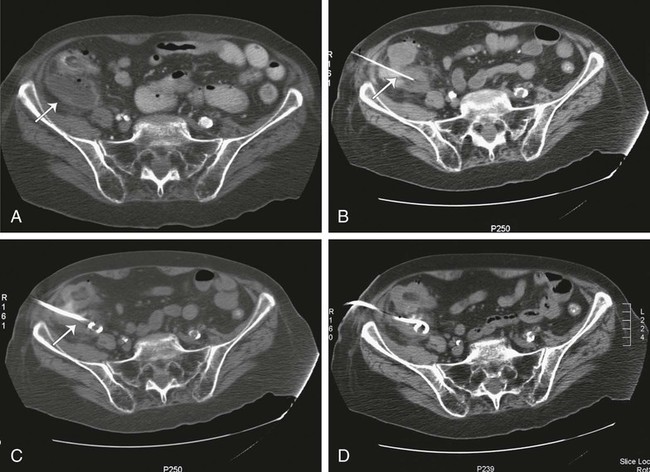
Since its introduction in the early 1980s, percutaneous abscess drainage (PAD) has become the primary drainage procedure for most infected abdominal fluid collections. Numerous large clinical series have demonstrated the efficacy and safety of this procedure.1–6 Image-guided drainage allows rapid, minimally invasive drainage of collections that had previously required surgical drainage and necessitated a trip to the operating room, general anesthesia, and was therefore associated with higher morbidity. Benefits of PAD in most cases include avoiding general anesthesia, laparotomy, and prolonged postoperative hospitalization, with a resultant reduction in morbidity, mortality, and hospital costs. As PAD has become more widely used, indications have continued to expand. Currently, the vast majority of infected intraabdominal and pelvic fluid collections are considered amenable to percutaneous drainage. Prerequisites for PAD are presence of a fluid collection and one or more of the following: suspicion the collection is infected, need for fluid analysis, or suspicion the collection is producing symptoms that warrant drainage.7 PAD may be performed in essentially every organ of the abdomen and pelvis. Liver abscesses, pancreatic abscesses, renal and perirenal abscesses, enteric abscesses, anastomotic abscesses, subphrenic abscesses, splenic abscesses, and pelvic abscesses, as well as subcutaneous abscesses and abscesses within musculature (e.g., psoas muscle) are examples of abscesses that have been successfully drained with percutaneous techniques. The goal of treatment can be complete cure of an infected fluid collection, or PAD may be used as a temporizing measure before definitive surgical treatment. For example, patients with diverticulitis and abscess formation would require, in addition to surgical drainage, a colostomy and subsequent takedown of the colostomy if operated on in the acute period. With PAD, the abscess and any existing or potential acute sepsis are treated first, allowing time for the acute inflammation to subside and ensuring a cleansed bowel at the time of resection so a primary anastomosis can be performed without colostomy. In other instances, PAD can serve as a palliative procedure for patients who are not candidates for curative surgical treatment. Contraindications to PAD are relative and should be weighed carefully against the suitable surgical alternative and the alternative of no procedure. Relative contraindications include coagulopathy that cannot be adequately corrected, phlegmons or collections containing a large amount of debris, hemodynamic instability such that the procedure could not be completed, inability of the patient to be positioned or cooperate adequately for the procedure, known adverse reaction to contrast material when administration is deemed necessary for success of the procedure, lack of a safe access route for drainage of a collection, and severely compromised cardiopulmonary status in patients undergoing procedures in which there is risk of further cardiopulmonary compromise inherent in the procedure.7 In reality, almost all these factors can be mitigated to some degree to allow abscess drainage. For example, most coagulopathies can be corrected to within an acceptable range of risk. In truth, the only absolute contraindication would be absence of a safe percutaneous route to the abscess. However, this is rare with the many options available to the interventional radiologist, such as angling of the computed tomography (CT) gantry, using an angled ultrasound approach, imaging after repositioning the patient, or instilling saline to displace structures at risk. The risks and benefits of PAD must be carefully evaluated in each patient. Decisions regarding whether to pursue abscess drainage in critically ill patients are best made in concert with the surgical and medical teams caring for the patient. After establishing the presence of a drainable fluid collection, several issues regarding patient preparation must be addressed before performing PAD. First, adequate antibiotic therapy is initiated if there is clinical evidence of infection. In the majority of cases, the team caring for the patient has begun antibiotic therapy before consulting radiology for potential PAD. In the rare instance in which antibiotic therapy has not been initiated, broad-spectrum antibiotics are administered before drainage. Antimicrobial therapy is not withheld for fear of sterilizing cultures; because the interventional manipulation can seed the bloodstream with bacteria, the risk of sepsis is too high.8 Coagulation factors, including prothrombin time (PT), partial thromboplastin time (PTT), and platelet count, are checked before intervention. Any coagulopathy is then corrected to the degree possible before performing the procedure. The catheter may be placed by either the trocar or Seldinger technique. Both are viable options and offer advantages and disadvantages in different clinical settings. Although each technique has its proponents and detractors, no randomized trial has been conducted to compare them, and the choice of which to use comes down to operator preference, which itself is usually a function of experience. The trocar technique is generally performed in conjunction with a guiding needle (Fig. 127-1). As discussed earlier, the guiding needle is usually 18 to 22 gauge and variable in length, depending on the depth of the collection. The guiding needle is placed in the collection under CT guidance. Once needle placement within the collection has been confirmed with imaging, the needle acts as a guide for catheter placement. Before placing the catheter, a sample of fluid can be aspirated and sent for laboratory analysis. This initial aspiration can be helpful in charactering the fluid. If no fluid is aspirated, the collection may be very viscous and thus suggest the need for a larger catheter. Alternatively, if the fluid is not grossly purulent and the collection is not thought to be infected, a Gram stain can be performed if the decision of whether to place a catheter will be influenced by these results. With the Seldinger technique, initial access can be performed with a needle under ultrasound or CT guidance (Fig. 127-2). When needle position within the cavity is confirmed by imaging, a wire is placed through the needle into the cavity. The needle is then removed over the wire, and the tract is serially dilated to the appropriate size to allow placement of the catheter. Two different types of needles can be used for initial access. “One-stick” systems use a 21- to 22-gauge needle that accepts a 0.018-inch wire. A three-component device containing a sheath, dilator, and stiffener is placed over this wire. With removal of the stiffener and dilator, a 0.035- or 0.038-inch working wire can be placed through the sheath. Further dilation can then be performed to place the catheter over this wire into the collection. Alternatively, a larger needle that accepts a 0.035- or 0.038-inch wire, such as a Ring needle, can be placed. The Seldinger technique is helpful when the window to the collection is narrow. This technique offers the further advantage of guiding the catheter into optimal position with the use of directional catheters under fluoroscopic guidance. The disadvantage of this technique is primarily that it can be more time consuming than the trocar technique. This time consideration can be particularly important with critically ill patients. Additionally, serial dilation can be painful even in well-sedated patients.
Percutaneous Abscess Drainage Within the Abdomen and Pelvis
Indications
Contraindications
Technique
Anatomy and Approach
Technical Aspects
Method

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Percutaneous Abscess Drainage Within the Abdomen and Pelvis