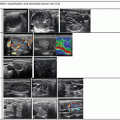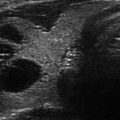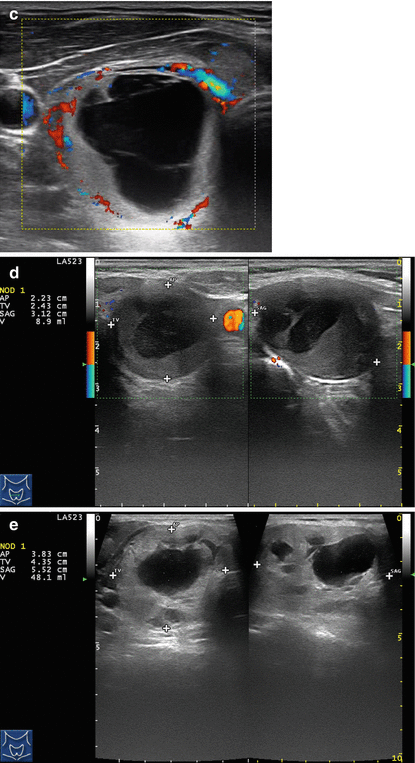
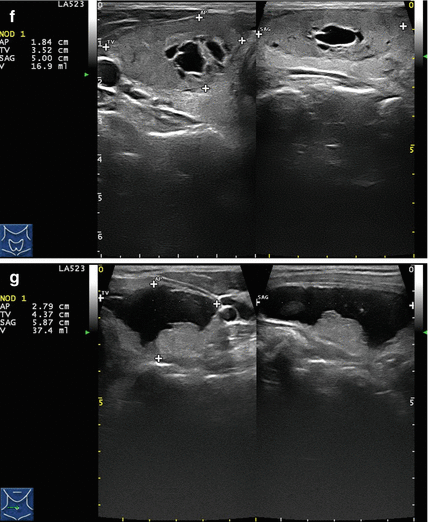
Figure 14.1
Types of cystic and mixed thyroid nodules: (a) a unilocular, “pure” thyroid cyst of the left lobe that appears anechoic (B-mode US, transverse view); (b) a multilocular (in this case bilocular), complex thyroid cyst of the right lobe with an internal septation (power Doppler US, transverse view); (c) a right thyroid cyst with solid and vascularized wall (color Doppler US, transverse view); (d) a left, hemorrhagic thyroid cyst (color Doppler US, transverse view on the left, sagittal view on the right); (e) a right, mixed thyroid nodule 50% solid, 50% cystic (B-mode US, transverse view on the left, sagittal view on the right); (f) a right, predominantly solid, mixed thyroid nodule (B-mode US, transverse view on the left, sagittal view on the right); (g) a left, predominantly cystic, mixed thyroid nodule (B-mode US, transverse view on the left, sagittal view on the right). All these types of thyroid nodules, except from the predominantly solid mixed type (f), are good candidates for PEI
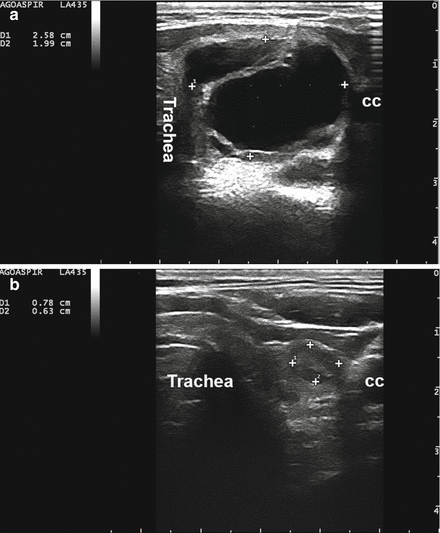
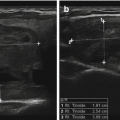
Figure 14.2
A complex thyroid cyst (calipers) before (a) and 6 months after (b) percutaneous ethanol injection. cc, common carotid artery
Clinical studies focused on PEI treatment of thyroid cystic nodules have consistently demonstrated that alcohol ablation achieves a significant volume reduction, ranging in most series between 65% and 90%. Accordingly, the procedure is expected to attain a ≥50% reduction of thyroid cyst volume in the large majority (70–95%) of patients [14, 21, 22, 25–33]. The consistency of PEI results is supported by reports from numerous different centers and authors over the last two decades, notwithstanding minor variations of the technique [14, 21, 22, 25–33] (Table 14.1). The preference of alcohol versus other sclerosing agents, e.g., tetracycline, polydecanol, arginine hydrochloride, and sodium tetradecyl sulfate [34–37], resides in its safety and affordability: the cost of the material is negligible, and the procedure may be repeated several times, if needed. In addition to measuring the cyst volume before and after ethanol instillation, the outcomes of PEI may be evaluated on clinical ground. Amelioration of pressure symptoms and cosmetic complaints is the rule, being recorded in about 75–95% of patients treated with PEI [24]. The measurement of the volume of the lesion before and after treatment provides an objective parameter, while the clinical outcome usually relies on subjective evaluations. In a recent Spanish study, a comprehensive ten-item questionnaire investigating goiter symptoms was proposed to the patients: each item was defined by a 1–5 score, and a final sum was calculated [38]. In another recent study from Korea, subjective pressure symptoms were rated on a 10-cm visual analog scale by the patients, while a 1–4 grade cosmetic score was attributed by the physician [33].
Table 14.1
PEI sclerotherapy in thyroid cystic nodules: clinical outcome
Patients | Mean follow-up (months) | Mean volume at baseline (mL) | Number of PEI sessions | Mean volume reduction (%) | Success rate (%)a | |
|---|---|---|---|---|---|---|
Yasuda et al. [13] | 61 | 6 | n.r. | 1–3 | n.r. | 72.1 |
Monzani et al. [21] | 20 | 12 | ~12.0 | 1–2 | n.r. | 95 |
Verde et al. [14] | 32 | 12 | 14.5 (1.5–65.8) | n.r. | 71 | 75 |
Antonelli et al. [22] | 26 | 12 | 16.8 ± 9.9 | 1–5 (mean 2.5) | n.r. | 77 |
Zingrillo et al. [30] | 43 | 37.0 ± 14.0 | 38.4 (4.8–166) | 1–4 (mean 1.5) | 91.9 | 93 |
Cho et al. [40] | 22 (13/9) | 3.5 (1–10) | 13.0 (3.5–42.0) | 1–6 | 64.0 | 68.1 |
Del Prete et al. [27] | 98 | 120 ± 14 | 35.2 ± 20 | 1–4 (mean 1.8) | 59.9 | 93.8 |
Kim et al. [29] | 20 | 4.4 (1–6) | 15.7 (12.0–48.6) | 1–3 (mean 1.8) | 64.0 | 65.0 |
Bennedbaek et al. [25] | 33 (26/7) | 6 | 8.0 (5.0–14.0) | 1–3 | 100b | 82.0 |
Valcavi et al. [26] | 135 | 12 | 19.0 ± 19.0 | 1–3 | 85.6b | n.r. |
Guglielmi et al. [28] | 58 | 82 | 13.7 ± 14.0 | 2.2 ± 1.3 | 86.6 | 86.2 |
Lee et al. [31] | 432 | 36.5 ± 12.9 | 15.6 ± 12.6 | 1–7 (mean 2.3) | 66.1 | 79.4 |
Kanotra et al. [32] | 40 (24/16) | 13.8 ± 5 | 12.2 (5.8–18.5) | 1–3 (mean 1.5) | 70.0 | 85.0 |
Sung et al. [33] | 36 | 17.0 ± 7.46 | 13.8 ± 11.9 | 1–2 (mean 1.2) | 93.0 | 94.4 |
217 | 6.0 ± 5.6 | 15.7 ± 18.1 | 1–3 | 85.2 | 90.3 | |
In et al. 2015 | 62 | 14.1 (12–24) | 7.2 mL (1.3–54.1) | 1–2 | n.r. | 81.3 |
Reverter et al. [38] | 30 | 2.1 ± 1.4 | 18.2 ± 15.5 | 1–3 | 85.9 | 100 |
Suh et al. [44] | 107 | 17.1 ± 12.2 | 20.8 ± 31.1 | 1–2 | 61.6 | 61.7 |
Three major determinants are known to negatively impact the success of the procedure: (a) an exceedingly large volume (e.g., ≥40–50 mL) of the lesion; (b) a mixed instead of a “pure” anechoic cystic structure; and (c) a thick and dense, as opposed to a watery, content of the cyst. The first two potential limitations, both likely to preclude a thorough and homogeneous alcohol diffusion within the lesion, are easily foreseen, while the third one, which stands as the main obstacle to the drainage phase, cannot always be anticipated before performing the procedure. In order to avoid this latter issue, the viscosity of the cystic content is usually assessed during the diagnostic fine needle aspiration which precedes the PEI procedure. This information is used to select the optimal needle gauge for aspiration. As a general rule, US evidence of echoic particles which, under the gentle pressure of the probe, float across the anechoic space like snowflakes under a gust of wind (“snow-globe” effect) is a reliable index of a watery, quite fluid content of the lesion, likely to allow an easy and complete drainage. Alternatively, when the fluid content is highly viscous, there may be insufficient withdrawal of material to ensure an adequate volume of the ethanol reinstillation within the lesion [39].
Very large cystic nodules may represent a challenging target because the PEI procedure usually turns out to be more difficult on practical ground: longer time is required for the drainage as well as the instillation phases to be carried out, and the compliance of the patient may be compromised by the prolonged duration of the procedure. Furthermore, an even and complete distribution of the ethanol on the inner surface of the cyst may not be optimally accomplished. Nevertheless, according to the available evidence, an inverse correlation between cyst volume and success rate of PEI procedure has not been conclusively demonstrated [25, 30–32, 40]. Far more convincing and concordant among different studies are the evidence collected on the role of the nodule structure as a major factor determining the final outcome of PEI treatment. While “pure” cystic nodules may achieve an impressive (>90%) volume decrease, similar results are usually not achieved in case of “mixed” nodules [39, 41–44]; and the latter are also more likely to require multiple PEI sessions. In order to achieve a successful treatment of this kind of lesion, a two-step approach has recently been proposed, based on an initial PEI session focused on the cystic component and a second PEI procedure performed 1 month later on the solid portion of the nodule [45].
Alcohol Ablation of Thyroid Cystic Lesions: The Technique
The patient is requested to lie in a supine position, with a pillow placed under the shoulders in order to ensure an adequate hyperextension of the neck. The neck area is prepped with an antibacterial/sterilizing solution; a sterile blanket is deployed over the patient’s chest; sterile gloves are worn by the operators; and the use of sterile gel and probe covers are utilized during US-guided procedures. The patient’s eyes may be sheltered from the risk of accidental contact with alcohol by a pair of protective glasses. Local anesthesia is optional and can be beneficial when larger (e.g., 16–18 gauge) needles are required. The selection of the needle size is a crucial step in order to ensure a fast and complete drainage of the fluid; as a general rule, most cystic lesions can be emptied using 20- to 23-gauge needles, and larger needles are to be reserved to extremely viscous lesions. At our hospital, we routinely use a 22-gauge spinal needle initially and move to a larger caliber stylus needle when s uggested by the circumstances (Fig. 14.3).


Figure 14.3
Equipment for the ethanol ablation of a thyroid cyst: (1) a 20-gauge spinal needle with stylet, (2) a 22-gauge spinal needle with stylet, (3) 2% lidocaine for local anesthesia (seldom necessary), (4) 95% sterile alcohol, (5) saline solution for washing the needle at the end of the procedure, (6) a 20 mL syringe for alcohol injection, (7) a 5 mL syringe for the administration of lidocaine in case of local anesthesia, (8) a pistol syringe holder for applying negative aspiration pressure, and (9) a flexible extension tube for connecting the hub of the needle to the tip of syringe
For didactical purposes, PEI is generally described as the succession of four phases: (a) insertion of the needle, (b) drainage of the cyst content, (c) injection of ethanol into the lesion, and (d) needle extraction. All the above listed phases should be performed under U S guidance, with a constant attention to the positioning of the needle tip (Fig. 14.4a, b). Sometimes, before PEI, pericapsular anesthesia with lidocai ne is performed (Fig. 14.4c).
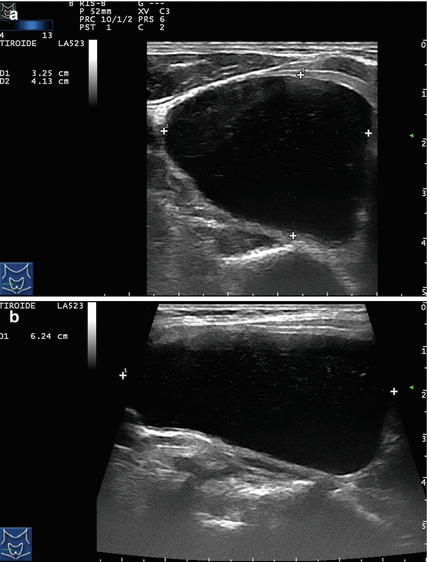
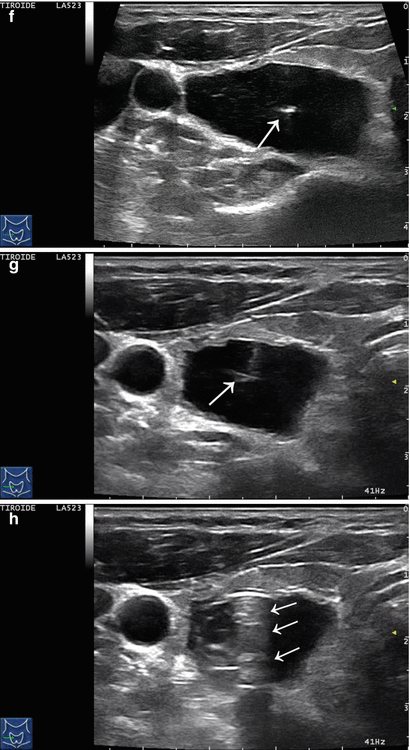
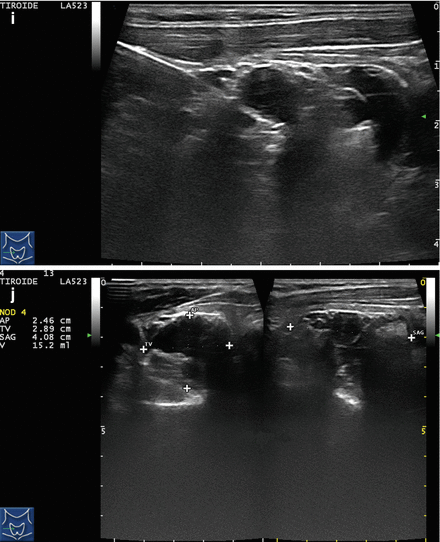




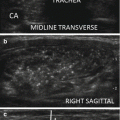
Figure 14.4
US-guided PEI of a thyroid cyst. (a) Basal US image of a right lobe thyroid cyst (B-mode, transverse view). (b) Basal US image of the right lobe thyroid cyst (B-mode, longitudinal view). (c) Pericapsular anesthesia with a 27-gauge needle (arrow). (d) Needle insertion into the cyst. (e) Aspiration of fluid content of the cyst (longitudinal view). (f) Aspiration continues and the cyst shrinks—tip of the needle (arrow)—(transverse view). (g) Thyroid cyst immediately before ethanol injection—tip of the needle (arrow)—(transverse view). (h) Ethanol insti llation—ethanol is visible as hyperechoic material (arrows). (i) The thyroid cyst is filled with an amount of ethanol equal to 50% of the aspirated liquid volume. (j) The thyroid cyst at the end of the procedure
Insertion of the Needle
In analogy with the different techniques for needle insertion suggested for FNA (see Chap. 12), this phase may be accomplished according to two basically different approaches: either the needle is inserted through a guiding device mounted on the US transducer, or it is directed “free-hand” into the lesion according to a perpendicular or a parallel approach. The needle should always be kept under visual control by gentle adjustments of the transducer (Fig. 14.4d), which can be held by the first operator or by an assistant as well. According to the reported outcomes, neither procedure can be claimed as clearly superior to the other: the adoption of a guiding device usually makes the operator more confident, and fast, throughout the injection phase, whereas the free-hand technique permits continuous repositioning of the needle along different spatial axes. It should also be considered that, once the needle has been inserted, the guiding device can be removed thus warranting a full-range motion of the needle.
Drainage of the Fluid Content
As soon as the stylet (if used) is removed, the fluid rapidly reaches the needle hub ready to spill out. A 20 mL syringe connected through a catheter (20–25 cm length) allows a smooth and effortless drainage of the fluid content and preserves the needle from the risk of being misplaced under the strain forces induced by traction as well by the deformation of the cyst along the drainage phase (Fig. 14.4e–g). The use of two devices may quite effectively facilitate this phase: a pistol syringe holder and a catheter connecting the needle and the syringe. Both devices contribute to easing the drainage of the fluid content, as well as the subsequent alcohol injection; at the same time, the connecting tube prevents the transmission of abrupt traction and strain movements to the needle.
It should be kept in mind that voluminous cysts may need to be emptied with more than 1–2 syringes, and all the potentially necessary materials and devices should always be at full disposal of the operators. In case of large lesions as well as dense, viscous cyst content and longer duration of the procedure may lead to the occlusion of the needle. This inconvenience may be obviated by reaming the needle with a stylet. Although the attention of the less experienced operators tends to be maximally focused on the insertion of the needle, while the drainage phase is usually perceived as less troublesome, this is quite a delicate step, likely to influence the full success of the procedure. Ideally, the fluid should be aspirated at a constant, slow speed, while keeping the needle tip under visual control: the progressive reduction of the cavity volume may frequently cause the needle tip to be plugged up by the cyst walls, which would require its gentle repositioning. In addition, an excessive energy applied to the suction may lead to capillary hemorrhage, causing a real-time refilling of the cyst. Operators facing the ascending phase of their learning curve should refrain from draining the fluid content to the last drop, as the visualization of the needle tip is easier if a small anechoic remnant of the cyst is left.
When the content of the cyst is made of viscous colloid not readily susceptible to drainage, a two-step approach is traditionally recommended: a small (e.g., 1–2 mL) volume of ethanol is injected with the aim of modifying the physicochemical properties of the material and the aspiration is postponed (1–4 weeks) [46, 47]. According to our experience, this strategy is almost invariably rewarding. Another, quite more cumbersome, approach is based on the use of a 16-gauge needle connected to a suction pump working at a 10–100 mmHg vacuum. In case the drainage is still ineffective, the 16-gauge needle may be substituted with an 8.5-French pigtail catheter. Once the fluid has been successfully drained, the ethanol is injected through the same device and after 10 min completely removed [48]. More recently, an open-window needle likely to drain quite dense material has been proposed for treating the most viscous cysts [49].
Ethanol Instillation
The injection of ethanol into the cyst should always be performed with extreme caution: the keystone which should guide this part of the PEI procedure is that the maneuver should be interrupted as soon as either resistance against further instillation is perceived by the operator in charge of injecting the alcohol, or the patient starts complaining of a sudden, burning pain. The access of the ethanol into the anechoic structure of the cyst is easily appreciated at US as a hyperechoic dense material refilling the anechoic space of the cyst (Fig. 14.4h). The lack of resistance to the injection and the progressive volume gain of the cyst indicate that ethanol is being correctly instilled inside the lesion. Similar as in the drainage phase, the prolongation of the ethanol instillation may cause the obstruction of the needle thus requiring the action of reaming. The central issue of the instillation phase deals with the calculation of the right amount of ethanol to be injected. As regards to this issue, there are no absolute rules to follow nor exact formulas to apply: in general, the quantity of ethanol required roughly corresponds in most procedures to the 50% of the total drained fluid (Fig. 14.4i). Taking this principle as a standard guideline, the operators should be prepared to adapt to the various circumstances and factors which may limit, or alternatively broaden, the volume of the instilled ethanol. In other terms, the onset of a major resistance to further injection, not amenable to be counteracted by repositioning of the needle tip, may require to stop the maneuver, irrespectively of the fact that the ideal quantity of ethanol has not yet been instilled. On the contrary, if the PEI procedure goes smoothly, with the patient fully compliant and the needle tip under optimal visual control, the amount of alcohol may exceed the theoretical target. Although the maximum amount of ethanol usually required for sclerotherapy of most thyroid cysts is <10–15 mL, larger volumes (e.g., up to 30 mL) can be occasionally used. Once the instillation phase is ended, two different modalities are available: (a) leave all the injected ethanol inside the lesion in order to permit the sclerosing properties of the agent to be exerted throughout the following days and (b) drain all the injected alcohol after a short while (e.g., 3–10 min). Apparently, these two alternative approaches share similar results and an identical safety profile [50]. The theoretical advantage of the latter strategy, likely to make the procedure slightly more complicated and time-consuming, resides in the reduced exposition of tissues to ethanol leakage. In addition, when dealing with a large cyst refilled with a conspicuous amount of ethanol, the reabsorption of the agent may be less than complete preventing a fully satisfactory reduction of the lesion size. Again, we would recommend avoiding dogmatic position in favor of or against each of the above-described techniques but rather try and verify both of them in the clinical practice in order to determine the most suitable and effective approach for each individual case.
Needle Extraction
Once the injection phase is ended, the needle should be rapidly extracted in order to minimize the contact of subcutaneous tissues with the tract of the needle dunked into the alcohol (Fig. 14.4j). This part of the procedure is usually the most painful for the patient but is fortunately transient, lasting usually less than 30 and sometimes up to 60 s: having the needle tip rinsed with a small amount of saline before being extracted may attenuate the subsequent transient, yet intense, pain. Systematic placement of an ice pack may reduce the risk of minor bleeding.
Side Effects
PEI of thyroid cystic nodules is a safe procedure: no major side effects are usually seen, and this evidence, consistently reported in all published series [24], is well asserted by the worldwide diffusion of this technique. Nevertheless, the relative safety of the procedure should not generate an excess of confidence in the operators: although the risk of recurrent laryngeal nerve (RLN) injury is exceedingly low, a maximum level of alertness should be always observed during the various phases of the treatment, and hazardous behaviors, such as the prolonged instillation of ethanol while there is a progression of resistance to the injection, should always be avoided. Normally, the integrity of the fibrous capsule of the cyst ensures that ethanol is fully contained within the lesion making its leakage into the perinodular tissues unlikely. Yet, even if every detail concerning the position of the needle, as well as the pressure transmitted back to the syringe, is in order, the occurrence of an intense pain suddenly signaled by the patient should prompt the operator to immediately stop the procedure. Only few data are available regarding factors possibly correlated to a higher risk of laryngeal nerve palsy: according to two studies, the larger the ethanol dose used, the higher the probability of dysphonia [25]. As previously mentioned, needle extraction is followed by a mild to moderate pain which typically lasts a minute and sometimes longer. Pain usually is described as radiating to the mandible angle or to the ear, and more seldom to the shoulder, to the chest or to the dorsal region. In order to alleviate painfulness associated with needle extraction, we suggest rinsing the needle with saline before extraction. On empiric basis, this simple expedient seems to some extent effective in reducing, although not completely preventing, the onset of pain.
Solid “Hot” and “Cold” Benign Thyroid Nodules
PEI was first been proposed for the tre atment of patients with autonomously functioning thyroid nodules (AFTNs) in the early 1990s [8–11, 51]. As reported by many published studies, mostly Italian groups, the initial results were quite encouraging. Successful outcomes both in terms of recovery of normal thyroid function and in terms of volume decrease (about 60–90%) of the lesion [8, 9, 11, 52–54] occurred in about nine out of ten of the treated subjects, with the best results achieved in patients with subclinical as compared to overt hyperthyroidism [54] smaller nodules (e.g., <20–40 mL volume) and in complex nodules, versus more reports of suboptimal outcomes when treating solid “hot” nodules [28, 55–57]. Unfortunately, PEI effects in AFTN proved to be only temporary with hyperthyroidism recurring in about 15–35% of the patients [58, 59]. This evidence is in line with the observation that studies based on thyroid scintiscan showed a persisting enhanced uptake by the nodular tissue in a large fraction (35–50%) of the autonomously functioning nodules treated with PEI [60, 61].
In addition to the disappointing results recorded by studies based on prolonged surveillance periods, two other major limitations have also transpired leading to a general loss of enthusiasm toward the utility of PEI in AFTNs. Firstly, treatment was time-consuming with multiple sessions (often as many as 5–10 sessions) of treatment required in the vast majority of patients. Secondly, at deep variance with the results of PEI applied to cystic nodules, PEI treatment of AFTNs was not devoid of important side effects. Laryngeal nerve palsy was recorded in most series, ranging from 0.7 to about 4.0% [53, 56, 59]. Moreover, a number of occasional serious adverse events (e.g., ipsilateral facial dysesthesia, transient Horner’s syndrome, septic complications, jugular vein thrombosis, and severe thyrotoxicosis) have also been reported [62, 63]. Multimodal therapeutic strategies based on the combination of PEI and radioiodine 131I and/or other interventional techniques have been proposed in patients with large AFTNs in more recent years in order to limit the therapeutic dose of 131I, with promising results [60].
PEI may achieve a significant volume decrease also in nonfunctioning solid thyroid nodules as shown by retrospective studies and a prospective randomized trial comparing ethanol injection and l-thyroxine (LT4) administration [31, 64, 65]. As for AFTNs , multiple PEI sessions are needed, and volume reduction is achieved at the expense of a not negligible rate of adverse events [6, 29, 66]. For this reasons, and in light of the expanding use of other interventional techniques (e.g., laser thermal ablation or radio frequency) likely to provide more consistent results, the role of PEI in the treatment of solid cold nodules is to be restricted to anecdotal situations, not susceptible to other, more effective treatment [17, 18, 24, 67, 68].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree