Median sensitivity (range)
Median specificity (range)
81% (48–90%)
53% (36–92%)
41% (27–59%)
94% [92–94%)
44% (26–73%)
89% (69–98%)
10% (2–17%)
94% (84–98%)
66% (33–100%)
43% (30–77%)
55% (17–84%)
80% (62–85%)
86% (78–91%)
48% (30–58%)
48% (33–84%)
92% (82–93%)
Echogenicity
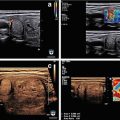
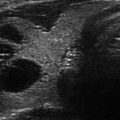
The echogenicity of a thyroid nodule refers to its brightness relative to the normal thyroid parenchyma. Normal parenchyma appears homogeneously hyperechoic or relatively bright on sonography as a consequence of the high number of acoustic interfaces within the normal follicles of the thyroid gland. The echogenicity of a nodule is described relative to this bright background of the normal thyroid as either (1) hypoechoic, meaning darker than the normal thyroid (Fig. 7.1a); (2) hyperechoic, meaning brighter than normal thyroid (Fig. 7.1b); or (3) isoechoic, meaning equal in echogenicity compared to the normal thyroid (Fig. 7.1c). Many nodules have regions of varying echogenicity and may be described by the dominant echogenicity (e.g.,, predominantly hypoechoic) or when there is no dominant pattern, as heterogeneous in echogenicity (Fig. 7.1d). For nodules that are partially cystic, the echogenicity of the solid part should be used to describe the nodule’s echogenicity, and cystic components are considered anechoic, meaning devoid of echoes, with hypoechoic used only in reference to solid components of a nodule [21] (Fig. 7.1e).
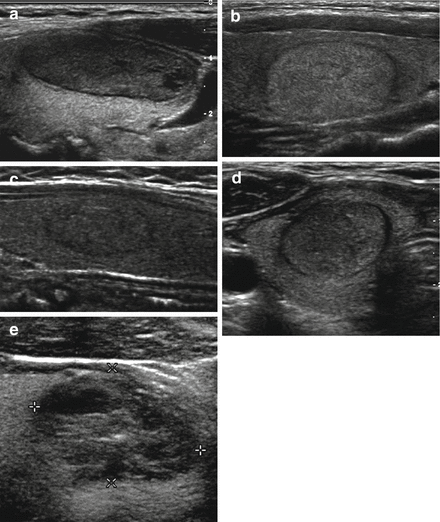

Figure 7.1
Nodule echogenicity. Images from ultrasound exams performed on different patients show (a) a hypoechoic nodule, (b) a hyperechoic nodule, (c) an isoechoic nodule, (d) a heterogeneous solid nodule with both hyperechoic and hypoechoic solid portions, and (e) a mixed cystic and solid nodule in which the solid component is isoechoic to the parenchyma. The nodules depicted in (b) and (d) proved to be follicular variant of papillary thyroid carcinoma, and the reminder had benign cytology
Most thyroid cancers appear dark, or hypoechoic, as compared with thyroid parenchyma. Histologically, the increased cellularity and cellular compaction present in classic papillary thyroid cancer and medullary thyroid cancer produce less acoustic interfaces than micro-follicles and therefore typically cause these lesions to appear hypoechoic compared with surrounding normal thyroid tissue [6–12, 14, 15, 17]. However, not all neoplasms of the thyroid are hypoechoic in appearance. Follicular neoplasms, including benign follicular adenomas, follicular carcinomas, and follicular variant of papillary cancers, are composed of small micro-follicles with variable amounts of colloid. Therefore, the echogenicity of these follicular-predominant neoplasms, both carcinomas and adenomas, is less commonly hypoechoic and instead is much more commonly isoechoic or hyperechoic compared with the parenchyma (Fig. 7.1b, d) [22, 23].
Additionally, many benign nodules will also appear hypoechoic. Since benign nodules are much more common than malignant nodules, a nodule that is hypoechoic but otherwise lacks any additional features associated with malignancy will statistically most likely be benign. Hypoechogenicity , as a unique characteristic of a nodule, is therefore only a moderately sensitive finding for malignancy, with a reported median sensitivity of about 80%. The reported specificity of hypoechogenicity for malignancy varies greatly depending on the histological subtypes of cancers in the population studied as well as the association of hypoechogenicity with other coexisting nodule features such as all solid consistency, calcifications, and central vascular flow. Some authors have identified a sonographic feature called “marked hypoechogenicity ” that describes a nodule with echogenicity that is darker than the neck strap muscles [13, 18, 20]. Compared to nodules that are hypoechoic with respect to parenchyma but not as hypoechoic as strap muscles, marked hypoechogenicity is less sensitive for identification of thyroid cancer but much more specific, typically over 90% [13, 18] (Fig. 7.2).
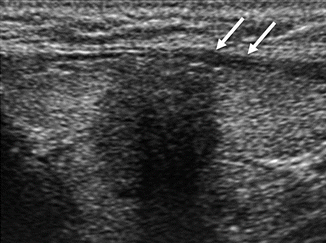

Figure 7.2
Marked hypoechogenicity . This nodule which proved to be a papillary thyroid carcinoma demonstrates echogenicity that is more hypoechoic than the strap muscles (arrows) overlying the thyroid
The assessment of echogenicity is subjective and tends to have only fair to moderate interobserver reproducibility even among expert observers (Kappa values 0.3–0.5) [16, 18, 24]. Echogenicity can be altered by differing sonographic techniques including changes in the overall gain and transducer frequency. Additionally, in patients with autoimmune thyroid disease such as Hashimoto’s thyroiditis , the echogenicity of the parenchyma is much more heterogeneous in appearance making classification of the nodule’s echogenicity more subjective.
Composition
Nodule composition describes the amount or proportion of solid soft tissue and fluid in a nodule. Nodules can be described as (a) solid, meaning composed entirely or nearly entirely of soft tissue with only a few small scattered cystic spaces (Fig. 7.3a); (b) predominantly solid, meaning that soft tissue components comprise at least 50% or greater of the volume of the nodule (Fig. 7.3b); (c) predominantly cystic, meaning that soft tissue components comprise less than 50% of the volume of the nodule (Fig. 7.3c); or (d) entirely cystic, meaning fluid filled without appreciable solid component (Fig. 7.3d). Some authors have used numerical percentages of cystic or solid elements to describe nodule composition, most commonly by quartiles [21, 25, 26].
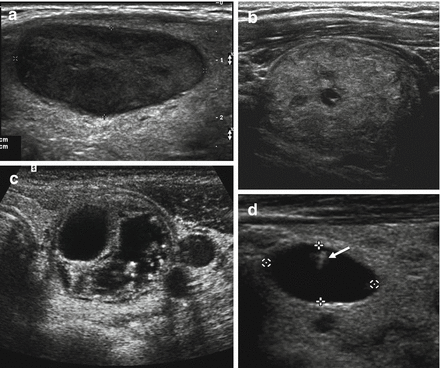

Figure 7.3
Nodule composition. Images from ultrasound exams performed on different patients show (a) an entirely solid nodule, (b) a predominantly solid nodule with scattered cystic spaces, (c) a predominantly cystic nodule, and (d) an entirely cystic nodule. Note the comet-tail artifact in this nodule (arrow)
Thyroid cancers are most commonly solid or nearly entirely solid and are more likely to be solid than benign nodules [9, 12, 14, 15, 20]. However, because benign nodules outnumber malignant ones, this feature carries a relatively low specificity for malignancy as an isolated feature. It is estimated that a solid nodule has a 15–27% likelihood of being malignant [25]. Although there is some subjectivity in reporting the degree of cystic change present in a nodule, the interobserver agreement for reporting solid nodule consistency is reported to be quite high [16, 18, 24].
Cystic change within a nodule is very common. Hyperplastic nodules contain abundant colloid which appears cystic on sonography, and neoplasms may undergo cystic degeneration or necrosis, producing cystic areas [27]. It is uncommon for cancers to have a predominantly cystic appearance, with only 6.1% of predominately cystic nodules being malignant [28]. The evaluation of a partially cystic nodule for malignancy risk should focus on an analysis of the solid component. Features concerning for a malignancy include an intralesional solid component that is hypoechoic and lobulated, has an irregular border, and/or contains calcifications (Fig. 7.4). From a recent Mayo clinic review of ultrasound findings in 360 consecutive patients undergoing thyroidectomy for thyroid cancer, only nine (2.5%) were more than 50% cystic, and of these, all except one had another suspicious ultrasound feature that included either microcalcifications, intranodular vascularity, a mural nodule, or a thick irregular wall surrounding the cystic area [28]. In addition, if the solid component is eccentrically (peripherally) located within a partially cystic nodule and the margin of the solid component has an acute angle with the wall of the nodule, the risk for malignancy is also increased. Conversely, if the solid component is isoechoic, is centrally located within the nodule, or is distributed concentrically, lacking an acute angle with the nodule wall, or has a smooth margin, the nodule is likely benign (Figs. 7.4 and 7.5) [29].
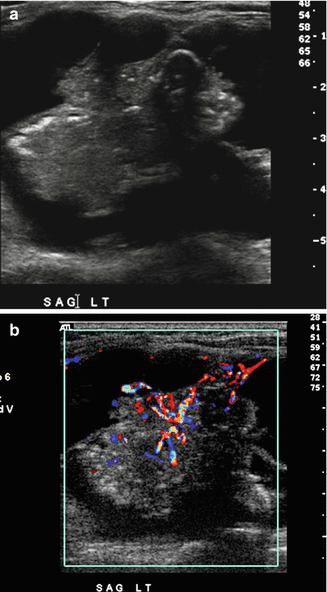


Figure 7.4
Cystic papillary cancer. (a) Grayscale and (b) color Doppler images of this cystic papillary thyroid carcinoma show the solid competent to be frond-like, lobulated, calcified, and have marked vascular flow

Figure 7.5
Partially cystic nodules . (a) The soft tissue component (solid arrows) of this predominately cystic nodule is eccentric and nodular and forms an acute angle with the wall of the nodule (dashed arrow) and proved to be a cystic papillary thyroid carcinoma. (b) The solid component of this cystic nodule is isoechoic to the parenchyma, with straight margins and spongiform in appearance, typical of a benign cystic nodule. (c) Two adjacent nodules (labeled N1 and N2) have a solid component that is central, concentric and smooth margin, typical of benign hyperplastic nodules
Pure cysts over 1.5–2 cm are rare, comprising <2% of all thyroid nodules, but if present, these are reported to always be benign [15] (Fig. 7.3d). In addition, a “spongiform appearance ” has a very low (<3%) risk of malignancy [30]. Moon et al. [18] defined a spongiform appearance as one in which the nodule has multiple microcystic areas that occupy more than 50% of the nodule volume and found that only 1 of the 360 thyroid cancers appeared spongiform in their series (Fig. 7.6). Bonavita et al. [19] defined spongiform to mean tiny spaces involving the entire nodule and found all 210 nonvascular nodules with this sonographic appearance to be benign. These spongiform nodules often have echogenic foci, generally linear, that are associated with the back wall of the small internal cystic spaces. These bright foci should not be confused with microcalcifications which are much smaller and punctate in appearance (Fig. 7.7). Often, comet-tail artifacts, which appear as an inverted triangle of parallel linear echoes, representing reverberation of sound waves caused by inspissated colloid [31], are present in both entirely cystic-appearing and spongiform nodules (Figs. 7.3d and 7.7b, c). It is worth noting that colloid may be present in both benign and malignant nodules. As noted above, very few thyroid cancers will be predominantly cystic, but these cystic carcinomas will typically have another suspicious US feature such as frond-like solid tissue and/or calcifications [28] (Fig. 7.4).
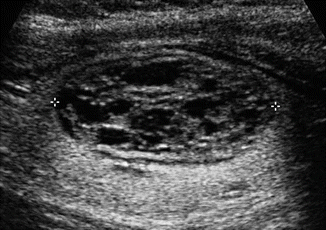
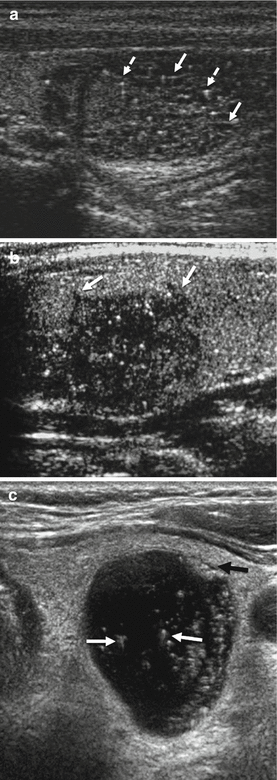

Figure 7.6
Spongiform nodule . This nodule has interspersed cystic spaces throughout, an appearance termed spongiform and is associated with a very low risk of malignancy

Figure 7.7
Echogenic foci. (a) Two types of echogenic foci are seen in this nodule. Some are short and linear and have comet-tail artifact (dashed arrows), whereas other are longer and linear (solid arrows) related to sound reflection from the back wall of the small internal cystic spaces in this spongiform nodule. FNA cytology is benign. (b) This nodule has microcalcifications which appear as punctate echogenic foci within the solid stroma of this hypoechoic solid nodule with irregular, jagged margins (arrows). FNA cytology is papillary thyroid cancer. (c) This nearly entirely cystic nodule shows multiple comet-tail artifacts (white arrows), which appear as inverted triangles of parallel linear echoes due to sound reverberation from colloid. An isoechoic solid component with smooth borders is seen anteriorly (black arrow)
Calcifications
Calcifications may be present in up to 30% of nodules and can be divided into different categories. Microcalcifications appear as small, punctate echogenic foci, less than 1 mm, and are more specific (in some studies, up to 96%) than sensitive for thyroid cancer [32]. Microcalcifications, which are thought to represent aggregates of psammoma bodies, are found in about 40% of papillary thyroid cancers and much less commonly in benign nodules and Hashimoto’s thyroiditis [32]. Due to the very small size of microcalcifications, they do not reflect the ultrasound beam sufficiently to cause distal acoustic shadowing, a feature dissimilar to larger or macrocalcifications. They most commonly are noted in nodules that have other malignant features such as all solid consistency, hypoechogenicity (often marked), and infiltrative margins, which help to distinguish them from other nonneoplastic and non-shadowing echoic foci in thyroid nodules (Fig. 7.7b). The interobserver agreement for the identification of microcalcifications is quite good [16]. Coarse or dense calcifications are larger than 2 mm and cause posterior acoustic shadowing (Fig. 7.8). Occurring within both benign and malignant nodules, these dystrophic calcifications are present in areas of fibrosis and tissue degeneration and necrosis. However, coarse calcifications, either associated with microcalcifications or appearing in the center of a hypoechoic nodule, may be worrisome for malignancy [15, 33]. Calcifications may also occur along the periphery of a nodule and can be thin and regular, often called “eggshell” calcification , which is most commonly noted in a benign nodule and less commonly in a malignancy [32] (Fig. 7.8c). Irregular or interrupted calcification is more concerning for a malignancy, and a particular worrisome finding is the interruption of this rim calcification by a soft tissue component of the nodule indicating probable invasion by the cancer (Fig. 7.8d, e).
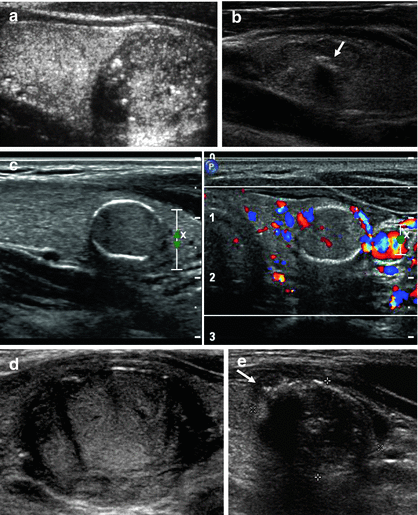

Figure 7.8
Macrocalcifications . (a) Hypoechoic solid nodule with both micro- and macrocalcifications. The macrocalcifications demonstrate posterior acoustic shadowing which appears as a dark area distal to the calcification due to complete reflection of sound by the large calcification. FNA cytology is papillary thyroid cancer. (b) A central linear calcification (arrow) is noted in this nodule. FNA cytology is benign. (c) A smooth peripheral rim of “eggshell” calcification is noted as well as central flow. FNA cytology is follicular variant of papillary carcinoma. (d) Note the interruption of the anterior calcified border that corresponded with localized invasion into the surrounding thyroid. FNA cytology is follicular variant of papillary carcinoma. (e) Note the extension of hypoechoic soft tissue with microcalcification beyond the peripheral rim of calcification. FNA cytology is papillary carcinoma
Margins
Sonography performed with high-frequency, high-resolution transducers allows detailed assessment of the interface of thyroid nodules with the surrounding parenchyma. The margins of a nodule can be defined because of either a difference in echogenicity between the nodule and the thyroid parenchyma or a border that demarcates a nodule when it is similar in echogenicity to the background thyroid. If the thyroid parenchyma has a normal homogeneous hyperechoic appearance, a hypoechoic nodule is easily identified, but detection of a hypoechoic nodule can be more challenging if the parenchyma is heterogeneous such as with Hashimoto’s thyroiditis. Certain margin features have been associated with thyroid malignancy. Infiltrative, spiculated, and/or jagged margins as well as lobular borders (Figs. 7.7b and 7.9) are concerning for an unencapsulated, invasive thyroid carcinoma. However, the distinction of infiltrative borders from indistinct borders is important because many small hyperplastic nodules will have poorly defined or indistinct margins at the interface between the nodule and the adjacent normal tissue, but these are not infiltrative and not considered to be a sign of a malignant growth pattern [18]. Interobserver variability for assessment of nodule margins carries the poorest agreement [16], likely reflecting the subtlety of this analysis. Additionally, studies did not distinguish between poor-defined and more aggressive margin features which may account for its reported lack of association with malignancy in several studies.
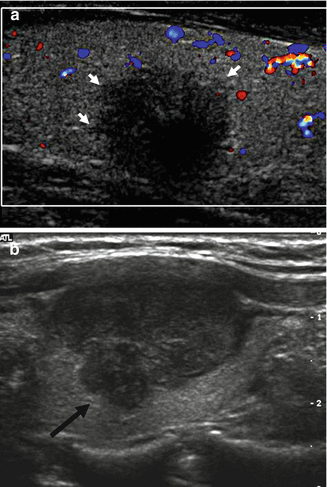

Figure 7.9
Nodule margins. (a) Hypoechoic solid nodule with jagged and spiculated margins (arrows) extending into the adjacent parenchyma. FNA cytology is papillary thyroid cancer. (b) Hypoechoic solid nodule with a lobulated margin (arrow). FNA cytology is papillary thyroid cancer
Nodules may also demonstrate the presence of a halo, defined as a sonolucent ring that surrounds a nodule. This generally forms the margin for iso- and hyperechoic nodules. Since benign hyperplastic nodules grow slowly and displace and compress the surrounding blood vessels as they expand, this may produce a thin halo, which demonstrates the nodule’s peripheral vascularity on color flow Doppler, and is found in about half of benign nodules (Fig. 7.10a, b). However, a halo can also be thick, irregular, and avascular [11] and may signify the presence of a fibrous capsule surrounding a neoplastic growth, either follicular or Hürthle cell, and is therefore more concerning (Fig. 7.10c).
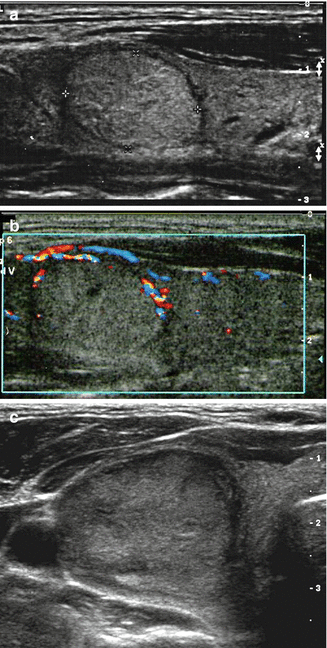

Figure 7.10
Halo. (a) Grayscale image of isoechoic nodule with thin regular halo. Cytology is benign. (b) Color flow Doppler image of the same nodule indicating the halo corresponds with peripheral vascularity. (c) Thick, irregular, and incomplete halo surrounding solid iso- to hyperechoic nodule. Histology is Hürthle cell cancer
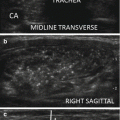
When evaluating a known or potential thyroid malignancy, it is important to assess for invasion beyond the thyroid to the adjacent soft tissues. Ultrasound may detect extrathyroidal extension when the tumor growth extends through either the anterior or posterior thyroid capsule, which normally appears as a bright white outline surrounding the thyroid. In such instances, the margin of the tumor has an ill-defined edge that interrupts this capsule [34] (Fig. 7.11). Only rarely is intratracheal growth demonstrated by sonography.
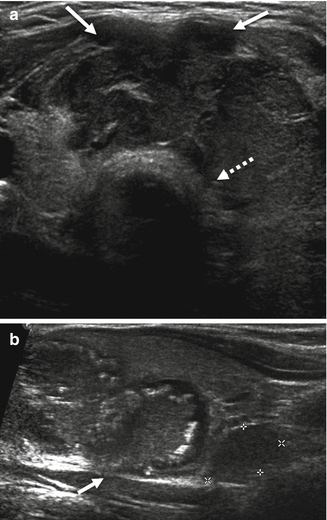

Figure 7.11




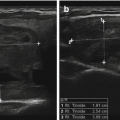
Extrathyroidal extension . (a) This anaplastic carcinoma shows clear extrathyroidal extension anteriorly (solid arrows) with tumor extending through the anterior capsule. Posteriorly there is lack of a clear margin with the adjacent trachea (dashed arrow) which proved to be tracheal invasion during surgery. (b) Sagittal image of the thyroid shows a calcified papillary thyroid carcinoma occupying much of the lobe. Posteriorly, there is discontinuity of the posterior capsule (arrow) which proved to be extrathyroidal extension of the tumor at surgery. Additionally, a metastatic central compartment lymph node is present (marked by electronic calipers)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree