Based on Surveillance, Epidemiology, and End Results studies, most renal cancers are low grade and slow growing. Long-term, single-center studies show excellent outcomes for T1a renal cell carcinoma (RCC), comparable to partial nephrectomy without affecting renal function and with much lower rates of complications. However, there are no multicenter randomized controlled trials of multiple ablative modalities or comparison with partial nephrectomy, and most studies are single-arm observational studies with short-term and intermediate follow-up. For treatment of stage T1a RCC, percutaneous TA is an effective alternative to surgery with preservation of renal function, low risk, and comparable overall and disease-specific survival.
Key points
- •
Based on Surveillance, Epidemiology, and End Results (SEER) studies, most renal cancers are low grade and slow growing. Since 1996, percutaneous thermal ablation (TA) techniques such as cryoablation, radiofrequency ablation, and microwave ablation have gained widespread acceptance for treatment of renal masses less than 3 cm in patients who are not surgical candidates.
- •
There are now long-term single-center studies showing excellent outcomes for T1a renal cell carcinoma (RCC), comparable to partial nephrectomy without affecting renal function and with much lower rates of complications.
- •
However, there are no multicenter randomized controlled trials of multiple ablative modalities or comparison with partial nephrectomy, and most studies are single-arm observational studies with short-term and intermediate follow-up.
- •
For treatment of stage T1a RCC, percutaneous TA is an effective alternative to surgery with preservation of renal function, low risk, and comparable overall and disease-specific survival. Ideally, randomized phase 3 trials should compare surgical resection with ablative techniques.
Introduction
Over the past 30 years, the recognition of the importance of renal function preservation has led to the development or adoption of several nephron-sparing treatment options of clinical stage T1a (<4 cm) renal masses, including (open, laparoscopic, and robotic) partial nephrectomy (PN), thermal ablation (TA), and active surveillance (AS). The procedure risk, underlying renal function is always weighed against the patients’ cardiovascular morbidity and mortality. Since initial case reports in 1996 and 1997, the adoption of TA for the treatment of clinical T1a renal masses has increased dramatically with favorable safety, efficacy, and preservation of renal function, as a minimally invasive alternative therapy for poor surgical candidates. TA has now evolved to become an alternative to surgery for treatment of clinical T1a renal masses in 2019 National Comprehensive Cancer Network guidelines.
More sophisticated understanding of the biology and natural history of solitary incidentally detected renal masses has led to an improved and less-invasive overall management paradigm. AS is now frequently recommended because of recognition of slow tumor growth rates, and wide prevalence of low tumor grade and low risk of adverse metastases. Overall, patient care and counseling have improved with a multidisciplinary approach.
Clinical considerations for TA therapy include patient age, comorbidities, and life expectancy as well as risk of developing renal failure or chronic kidney disease and the potential need for dialysis. In patients with multifocal or bilateral renal masses, or any positive family history of renal neoplasm, genetic susceptibility needs to be considered. , This article reviews the performance of different TA modalities and the goals of ablation therapy for treatment of renal cell carcinoma (RCC).
What is the image-guided thermal ablation?
Percutaneous image-guided tumor ablation refers to a group of minimally invasive treatment options using primarily ultrasound (US) and/or computed tomographic (CT)-guided needle-based thermal energy applicators to destroy focal malignancies. Over the past 24 years, these techniques have proliferated and become routine for treatment of renal tumors.
TA techniques eradicate malignant cells by inducing irreversible thermal cellular injury. In TA therapy, the total projected energy delivered to the target is proportional to the radius 3 of the lesion target. Thus, the overall required energy for small lesions (1–2 cm) is significantly less than for intermediate (2–3 cm) and larger (3–5 cm) lesions, fundamentally limiting the utility of TA for large lesions. The main goal of ablation procedures is to remove all viable malignant cells by treating the designated target and beyond into the visible tumor margin, typically the normal surrounding parenchyma up to 0.5 cm.
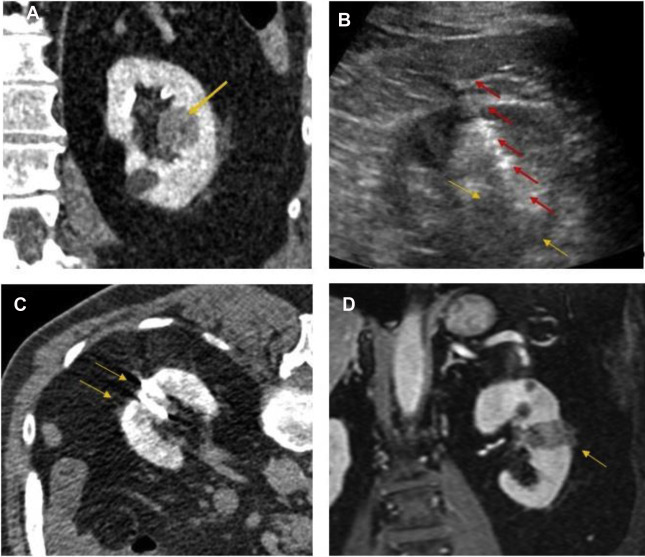
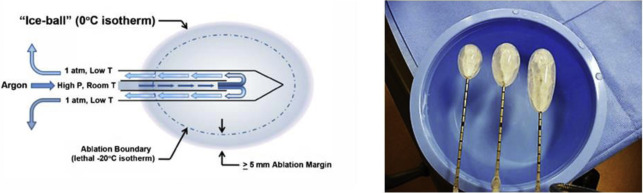
TA techniques for treatment of renal tumors are either cold (cryoablation) or heat (electromagnetic [ie, radiofrequency [RF], microwave, laser] or US) based. Cryoablation, first described for renal tumor ablation in 1996, induces cell death and apoptosis with a dual freeze-thaw cycle causing intracellular and extracellular ice formation (visualized as an “ice ball” on imaging). The goal is to reach to less than −140°C in the center of the lesion, −40°C at the margin of the lesion, and 0°C at the edge of the ice ball to create a near spherical ablation. Subsequently, the thaw cycle ruptures the osmotic cellular phospholipid membrane. The available cryoprobes are 13- to 17-G needles with internal shafts that circulate compressed argon gas, which produces dramatic cooling by dropping the gas pressure, called the Jewell-Thompson effect. Helium gas is used for the thaw cycle. An array of up to 8 probes can create a large fused ice ball to treat lesions up to 5 to 6 cm, generally much larger than radiofrequency ablation (RFA) or microwave ablation (MWA) ( Fig. 1 ).
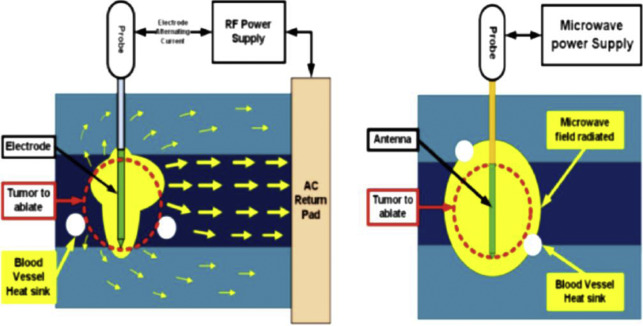
Heat-based ablation was once synonymous with RFA and was first described in 1997 for treatment of RCC. RF energy is an electrical impedance-controlled pulsed current, generally at 500 MHz, which induces tissue heating around straight or expandable multitined needle electrodes placed within the target. Current passes from the generator through the electrode into the target tissue and is grounded through pads on the patient’s skin, resulting in active frictional heating to greater than 60°C by rapidly oscillating water molecules at areas of high current density near the electrode, resulting in immediate coagulative necrosis of heated tissue. Nonelectric thermal diffusion (conductive heating) also occurs and results in a larger volume of cell death than beyond direct electric heating. Conductance is an important property of RFA, and tissue heating resulting in charring can decrease conductance, reducing effectiveness. A variety of RF needle designs include expandable multitined hooklike electrodes and straight internally cooled needles that allow for up to 3 probe placements simultaneously.
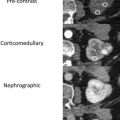
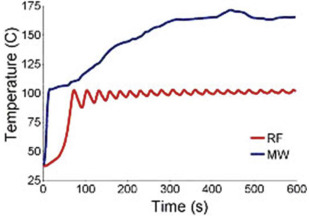
MWA, approved for use in the United States and used for RCC treatments since 2008, is a newer needle-based TA that heats target tissues to greater than 100°C because of more efficient frictional heating of water molecules by wavelike electromagnetic energy at either 915 MHz or 2450 mHz. Microwave energy is produced by generating dielectric hysteresis (rotating dipoles) at either 915 MHz or 2.45 GHz from the exposed tip of a needlelike probe (antenna) into surrounding tissue, resulting in more efficient and robust frictional heating of water molecules in tissue to greater than 100°C. Because of the wave property of MWA, permittivity is a useful metric determining efficacy, and marked differences in permittivity between tumors and surrounding tissue may allow better treatment with MWA. Unlike RFA, MWA is also not affected by carbonization or impedance and does not require grounding pads. In addition, diffusion of microwave energy is enhanced in neoplastic tissues because permittivity is generally greater in neoplastic tissues than in normal tissues. MWA is much less dependent on electrical conductivity of tissue because the energy delivery is less limited by the exponential rising electrical impedances of heated tissue in contrast to RFA. For successful ablation, the tissue temperature should be maintained in a range of 100°C to 120ºC to ablate lesion adequately and avoid carbonization around the needle tip because of excessive heating. The severity of the thermal damage depends on tissue perfusion, tissue temperature, and the duration of heating ( Figs. 2 and 3 ). MWA occurs more rapidly than RFA and cryoablation.
What are the important factors that can influence the result of the ablation?
The complete and adequate cell destruction by TA requires that the entire target and an ablative margin of 0.5 to 1 cm reach to the optimal cytotoxic temperatures. However, the ability to heat or cool the tissue, particularly for large volumes, is influenced by several factors like the amount of energy deposited (number of probes), tissue perfusion, the absolute temperature achieved at any point of target, and the heterogeneity of tissues.
Typically, TA times are based on the size and location of the lesions. Small exophytic renal lesions require the least time and energy, and large central lesions require the most time and energy because of low- and high-thermal dissipation, respectively, from surrounding heat sink inducing large central blood vessels and high and low insulation, respectively, from surrounding perinephric fat.
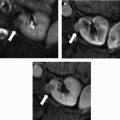
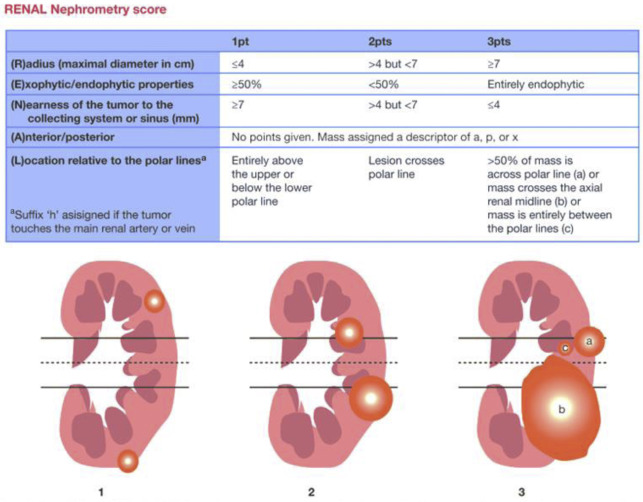
For treatment planning, the 5-parameter nephrometry score is useful for classifying the complexity of renal masses for PN and adapted for percutaneous ablation based on anatomic characteristics: tumor size, proximity to collecting system or renal sinus, and location (exophytic or endophytic, anterior or posterior, and orientation to polar lines). These parameters are for planning but are not associated with oncologic outcomes independently. McClure and colleagues first showed that higher nephrometry scores had a small adverse effect on efficacy of RFA, and this was later confirmed by Camacho and colleagues for RFA and by Ierardi and colleagues and Klapperich and colleagues for MWA. However, in our previous study this relationship was not found for MWA ( Fig. 4 ).
Preference and choice of ablation modality
Cryoablation is the dominant percutaneous TA treatment modality for T1a RCCs because of its efficacy and ability to clearly visualize the zone of ablation (ice ball) under CT or MR guidance. , The ice ball is the near spherical ablation zone with sharply demarcated margins encompassing the target renal mass with 5- to 10-mm surrounding margin so that the margin of the lesion is at the −40°C isotherm, whereas the center is ideally less than −140°C. This ability to visualize the ablation zone is not possible with many heat-based systems under CT guidance, but the echogenic cloud of ablation can be seen with US guidance. However, radiologists who are unfamiliar with US monitoring prefer the CT-based monitoring with the ice ball. Also, current systems can power up to 8 cryoprobes simultaneously, which increases volume and efficacy of cryoablation for treating both T1a and T1b renal masses. Like all TA modalities, cryoablation efficacy is limited by dissipation of cryoenergy (cold sink) from tissue perfusion by large arteries or veins. Large-volume cryoablation uniquely has a small but elevated risk for cryoglobulinemia or cryoglobulinuria, with associated risk of transient acute tubular necrosis. There is also an approximately 10-fold higher risk of hemorrhage relative to RFA and MWA. , Despite this, radiologist training, equipment preference, and experience are frequently the primary drivers of decisions regarding selection of TA type. Cryoablation is preferred for treating metastatic renal cancer lesions in the renal bed, adjacent to the spine or bowel, in the retroperitoneum, pleura, and subcutaneous tissue. , Other complication rates (besides bleeding) for cryoablation are similar to other ablation modalities.
Cryoablation, RFA, and MWA rely on skills of US and/or CT guidance to precisely place probes into renal lesions while avoiding adjacent normal tissues like vessels, bowel, renal pelvis, and ureter. Probes are placed into the deepest portion of the tumor initially and then retracted. RF and MW probes may have to be repositioned at opposite margins or may need to be circumferentially placed within larger tumors (>3 cm) to create larger overlapping zones of ablation. With cryoablation, up to 8 probes may be inserted within larger tumors. Continuous US monitoring during RF and MW ablation is important, because the operator monitors the rapidly forming heat generated release of water vapor and nitrogen, creating an echogenic cloud (“heat ball”), similar to the intermittent CT or MR imaging of the much slower forming ice ball of cryoablation. Both are surrogate markers of efficacy for TA.
The best marker of efficacy for any technique is to immediately perform a CT scan and/or MR imaging to see the predictable changes of TA. These changes include a smaller nonenhancing dense lesion on CT, T1 hyperintense or T2 hypointense lesion on MR imaging, and echogenic lesion on contrast US with a surrounding V-shaped nonenhancing region owing to thermal infarction of the segmental artery.
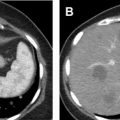
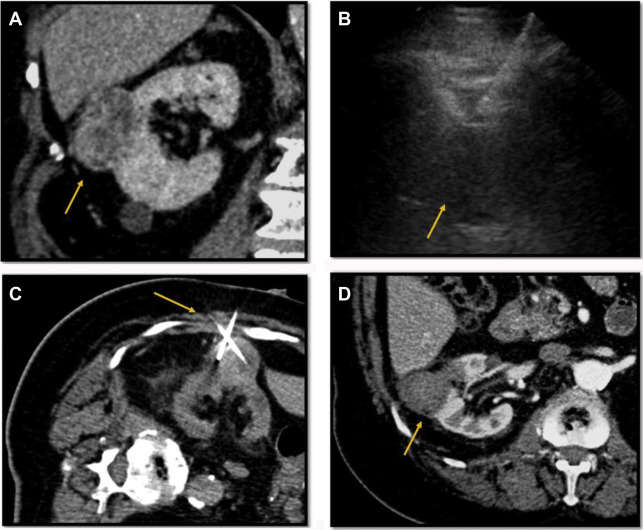
One of the limitations of RF ablation is related to carbonization of tissues adjacent to the electrode, which decreases conductivity and increases tissue impedance owing to high temperatures greater than 60°C. A variety of strategies have been developed to compensate, including water perfusion of the electrode shaft, multiple electrode placement with rapid switching, and slow ramp-up of energy delivery. RF ablation is less effective for tumors larger than 3 cm due to the large amount of energy required, centrally located tumors due to large heat sink, and tumors near the ureteropelvic junction of the collecting system due to heating-related stricture risk , ( Fig. 5 ). For larger lesions greater than 4 cm, a combination of embolization and RFA is more effective because energy dissipating tissue perfusion is eliminated or decreased. , ,
MWA is a faster and more efficient wave-based heating technique that relies on rapid heating of tissue water because of antenna and generator design and also properties such as wavelength, frequency, power, and cooling. MWA leads to much faster, hotter, and efficient heating of tissues than RFA. It is much less susceptible to heat-sink effects from adjacent renal vessels but can also cause faster and more severe thermal injury than RFA. Tumor size and location are also important factors because larger lesions and central lesions are more challenging to ablate for MWA albeit to a lesser degree than RFA. Ablation of tumors located close to the ureteropelvic junction and ureter increase risk of thermal injuries, such as stricture or leak or thermal injury to the collecting system. On average, fewer applicators are needed, and ablative margins are easier to obtain ( Fig. 6 ). Moreover, multiple studies have shown that MWA could effectively treat 5-cm lesions with similar efficacy to 3-cm lesions treated with RFA. , , Major complications of MWA occur in approximately 4% and include bleeding, infection, urine leak, stricture, and nontarget ablation, including skin burn if the target is within 3 cm of the skin. , ,