Chapter 9 Pericardial and Myocardial Disease
PERICARDIAL DISEASES
Anatomy
The inner visceral layer of the pericardium, also called serous pericardium or epicardium, is directly attached to the heart and gives a glistening appearance to the heart. The parietal pericardium is attached to the surrounding mediastinum, anteriorly to the superior pericardial sternal ligament, inferiorly to the central tendon of the diaphragm, and posteriorly to the esophagus and descending thoracic aorta. The fat outside the parietal pericardium is called pericardial fat. It is mediastinal fat that is adjacent to the pericardium. The fat inside the visceral pericardium is called epicardial fat, and it is directly attached to the cardiac chambers. The coronary arteries run within the epicardial fat. The visceral pericardium covers both, coronary arteries and epicardial fat. The pericardium does not cover only the heart but also extends about 3 cm into the root of the aorta and pulmonary artery where the visceral and parietal pericardium come together to form the pericardial reflection (Figure 9-1).
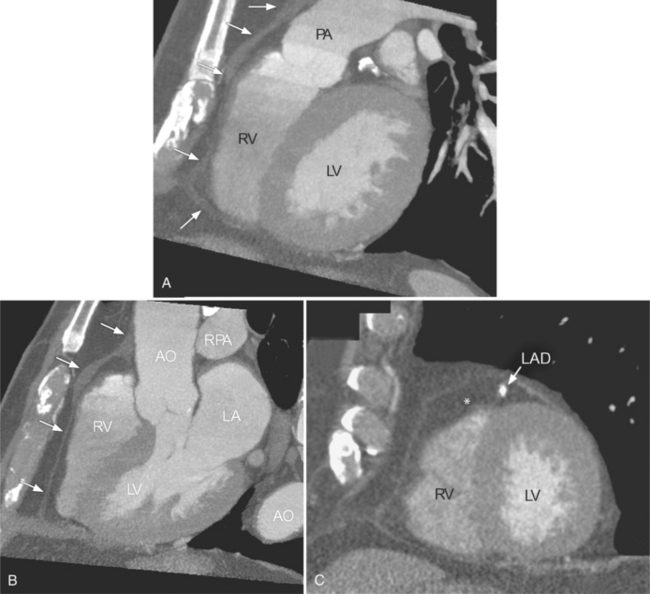
There are a few spaces inside the pericardial cavity in which fluid tends to accumulate and through which surgeons could stick their fingers or pull bypasses if they choose to do so—and sometimes they do. The superior pericardial recess is such a space. It connects the right side and the left side of the pericardial space between the root of the ascending aorta and the right main pulmonary artery (Figure 9-2). Sometimes fluid that has accumulated there may mimic aortic dissection, a thickened aortic wall from arteritis or enlarged lymph nodes. On rare occasions, a surgeon may opt to pull a venous bypass graft through the superior pericardial recess, if the graft is too short to be placed in the typical position anterior to the pulmonary artery (Figure 9-3).
Pericardial Effusion
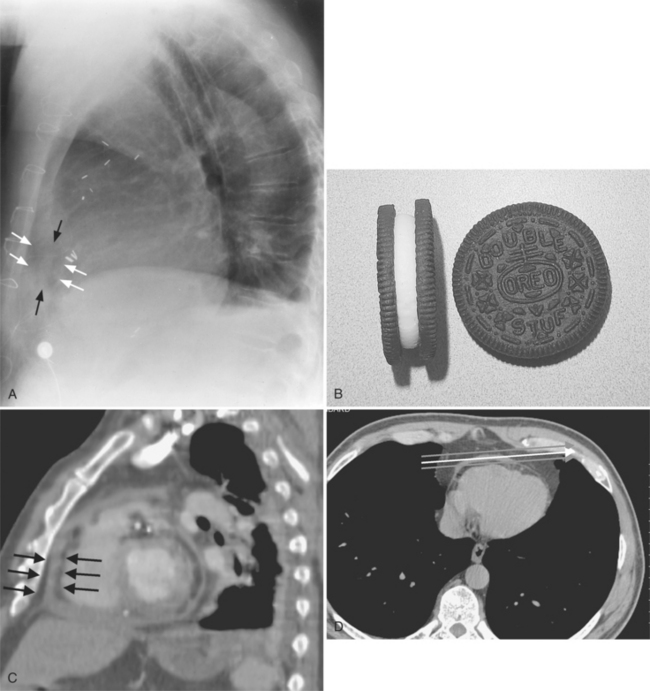
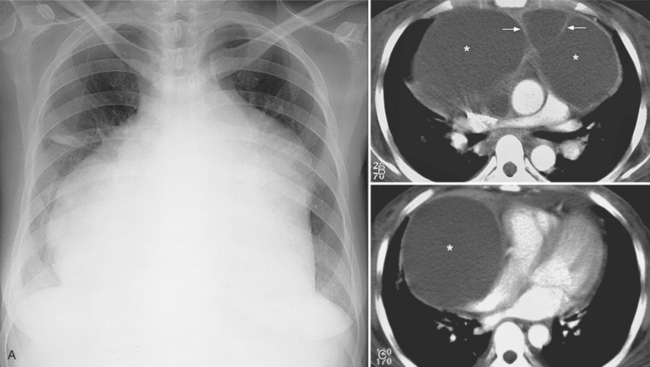
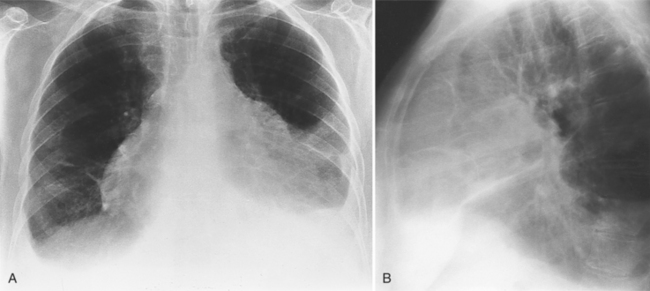
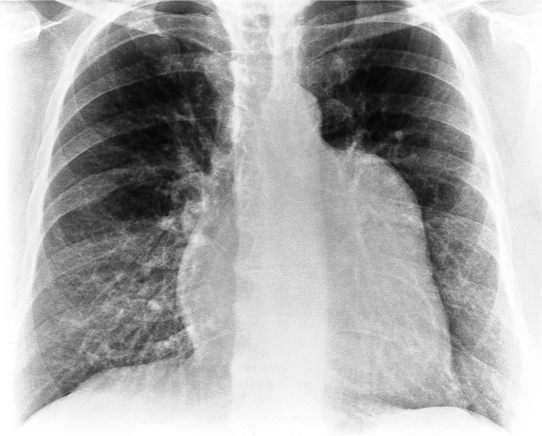
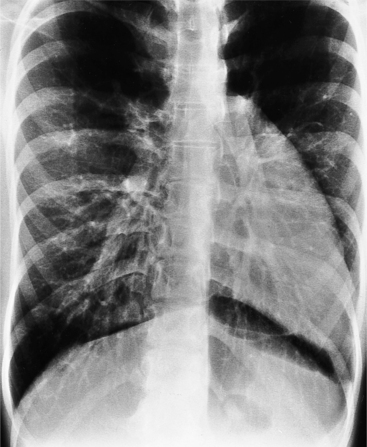
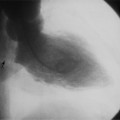
The normal pericardial space in the adult can be distended with 150 to 250 ml of fluid acutely before cardiac tamponade results. Cardiac tamponade is caused by excess fluid in the pericardial space, which compresses the heart and thus causes a low cardiac-output state. In tamponade, the cardiac size on the chest radiograph is slightly to markedly increased. The heart may have a water-bottle appearance in which both sides are rounded and displaced laterally (Figure 9-4). The differential diagnostic considerations for a water-bottle heart are global cardiomegaly, large anterior mediastinal mass, or pericardial effusion. If you are lucky, you may see the Oreo™ cookie sign on the lateral chest x-ray (Figure 9-5). In this sign, a radiolucent stripe behind the sternum (pericardial fat), then a more radiopaque stripe (pericardial effusion), followed by yet another radiolucent stripe (epicardial fat) will be noticed.
Appearance of Effusion on Computed Tomography and Magnetic Resonance Imaging
In MRI on spin echo sequences, pericardial effusions are of low signal intensity, which in part is from low protein content and from the motion of the fluid, which causes phase dispersion (Figure 9-6). Although T2-weighted images demonstrate high signal intensities for fluid in other areas of the body, they are not useful for characterizing pericardial fluid because the motion of the fluid causes signal loss. Gradient echo images depict any fluid in motion with high signal; therefore, pericardial effusion will be bright. Phase contrast images allow for quantification of flow and can be helpful in the characterization of pericardial fluid. The appearance of pericardial hematomas depends heavily on the type of hemoglobin present (e.g., oxyhemoglobin, deoxyhemoglobin, and methemoglobin).
Pericardial Effusion Syndromes
Infection, Collagen Disease, Metabolic Disease, and Tumors
Many infectious and metabolic diseases, tumors, radiation, drug reactions, and collagen disorders, such as systemic lupus erythematosus and scleroderma, typically cause small pericardial effusions. Uremic pericarditis occurs in about 50% of patients with chronic renal failure and is an indication for dialysis. Most effusions do not lead to cardiac tamponade. Common diseases that form pericardial effusions are listed in Box 9-1. Infectious agents that cause pericarditis with resultant effusions are usually coxsackievirus group B and echovirus type 8. Tuberculous pericarditis is uncommon except in patients with acquired immune deficiency syndrome (AIDS).
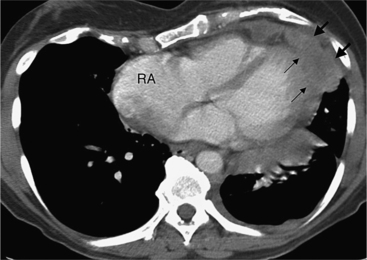
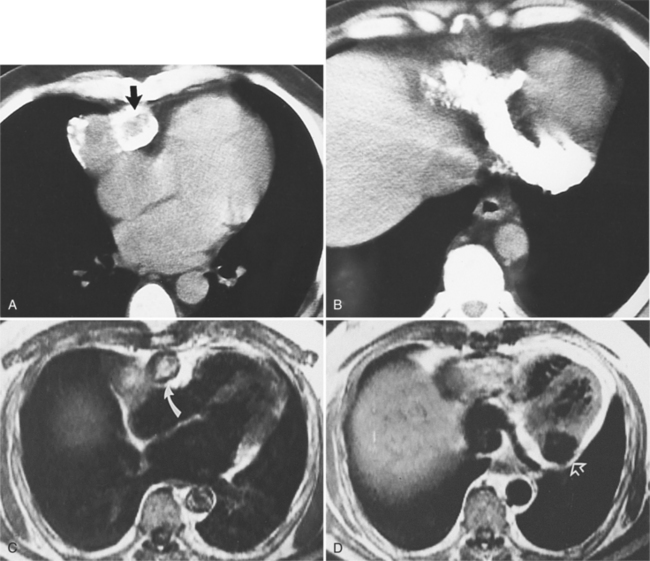
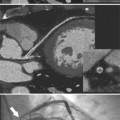
Although many bacterial, viral, or fungal agents can cause pericarditis, the most common organisms are Staphylococcus, Haemophilus influenzae, and Neisseria meningitidis (Figure 9-7). In addition to a hematogenous source, pericardial infections result from extension from a myocardial abscess related to infective endocarditis, from mediastinal abscess caused by fistula, and from carcinoma of the lung and the esophagus. A loculated pericardial fluid can represent hematoma, abscess, or lymphocele or may be secondary to fibrous adhesions from previous pericarditis. Loculated pericardial effusions can appear similar to pericardial cysts. Neoplastic pericardial effusions are usually related to systemic metastatic disease. The pericardium demonstrates nodular thickening with enhancement of the nodules. Infiltration of the epicardial or pericardial fat, myocardium, or adjacent vascular structures may be seen (Figure 9-8).
Myocardial Infarction (Dressler Syndrome)
The most common cause of pericardial effusion is myocardial infarction with left ventricular failure. An increase in either right or left heart pressure may also cause a pericardial effusion. About 5% of patients with acute myocardial infarction develop a pericardial effusion. Dressler syndrome is the development of pericardial and pleural effusions 2 to 10 weeks after a myocardial infarction (Figure 9-9). These effusions may be hemorrhagic and can result in cardiac tamponade, particularly if the patients have been given anticoagulant medication.
Constrictive Pericarditis
Constrictive pericarditis is caused by adhesions between the visceral and parietal layers of the pericardium. It occurs after pericarditis from any etiology but is more frequently ascribed to viral or tuberculous pericarditis, uremia with pericardial effusion, and after cardiac surgery. Dense fibrous tissue covers the outer surface of the heart, obliterates the pericardial space, and causes the thickening of the pericardial contour as seen on magnetic resonance and CT. Later calcification may occur. The fibrous adhesions prevent the valve plane from moving toward the cardiac apex in systole and therefore restricts diastolic filling of the heart. Effusive-constrictive pericarditis is a disease in which hemodynamic signs of constriction remain after a pericardial effusion has been aspirated (Table 9-1).
TABLE 9-1 Radiologic criteria for constrictive pericarditis
| Modality | Sign |
|---|---|
| Chest x-ray | Eggshell calcification of pericardium |
| Computed tomography | Thickened pericardium |
| Pericardial calcifications | |
| Magnetic resonance | Thickened pericardial contour imaging (>4 mm) in the absence of freeflowing pericardial effusion |
| Septal bounce on cine magnetic resonance images | |
| Pericardial adhesions proven by tagged cine magnetic resonance imaging | |
| Enhancing pericardium |
Calcifications
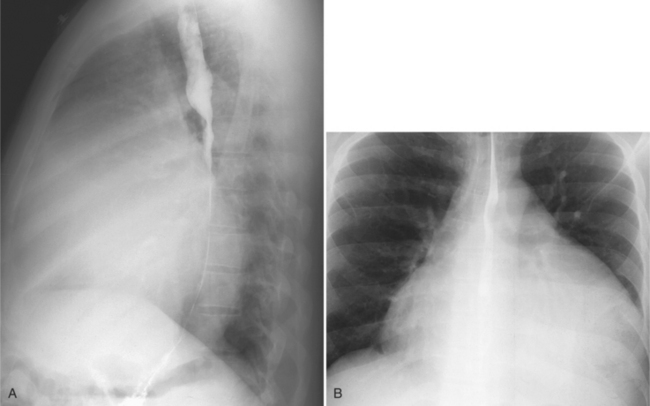
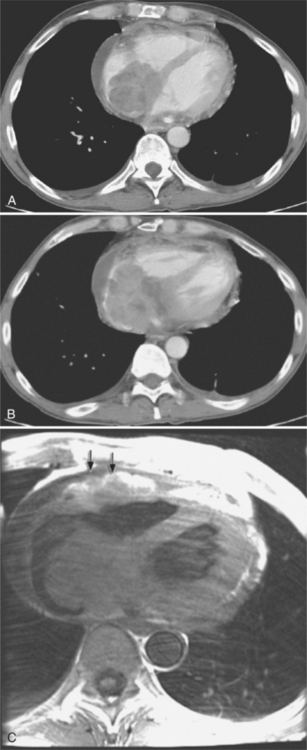
On the chest radiograph or CT, constrictive pericarditis may be suggested by the presence of pericardial calcification. The calcium may be quite thin and linear and appear as “eggshell calcification” around the margins of the heart (Figures 9-10, 9-11). Care must be taken to differentiate this pattern from the calcifications within the myocardium in old infarcts. The etiology of the pericardial calcifications in constriction is speculative, but it is seen mainly after viral and uremic pericarditis. A second type of pericardial calcification is a shaggy, thick, and amorphous deposition, which historically was rather specific for tuberculosis (Figures 9-12, 9-13). The calcium is particularly obvious in regions of the heart in which normal fat is found, namely in the atrioventricular grooves. Calcium in the atrioventricular region may indent the heart focally, producing “extrinsic” tricuspid and mitral stenoses. However, a calcified pericardium does not necessarily imply that constriction exists.
Comparison with Restrictive Cardiomyopathy
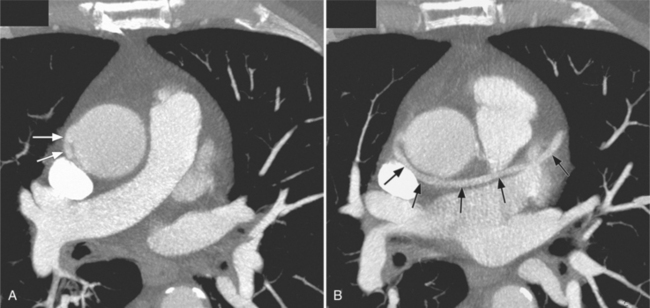
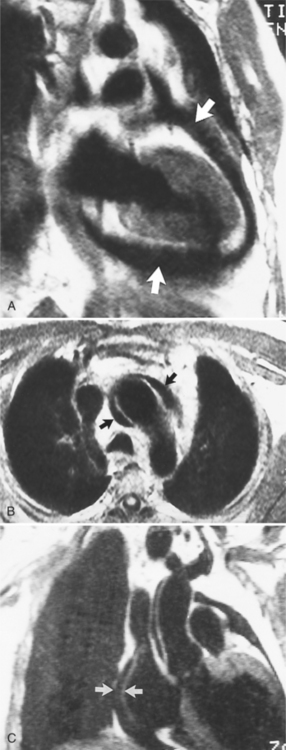
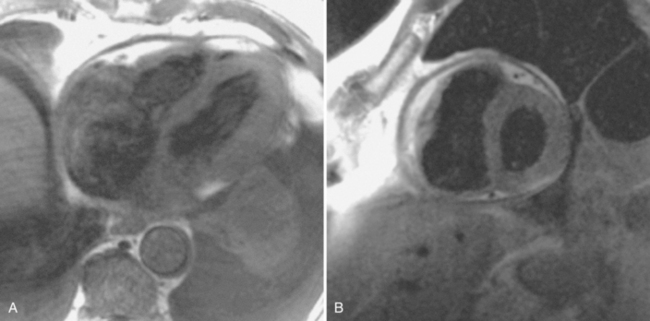
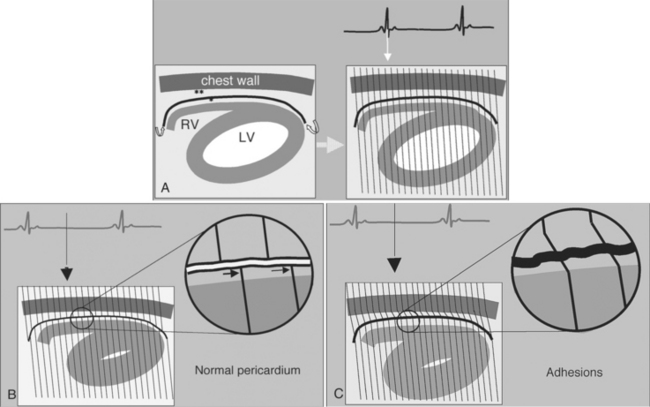
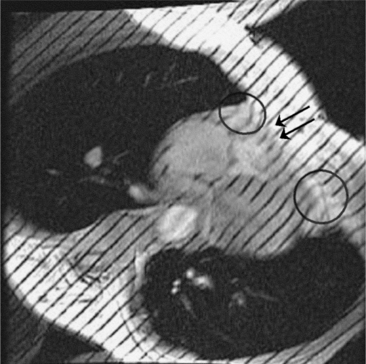
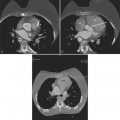
Constrictive pericarditis may be impossible to distinguish from restrictive cardiomyopathy based on hemodynamic tracings alone (see Table 9-1). Although MRI and echocardiography may show an abnormally thick pericardium, CT is the best imaging procedure to reveal calcified pericardium (see Figure 9-11). The pericardium in a restrictive cardiomyopathy does not calcify. Although the presence of pericardial calcium is strong evidence that a constrictive and not a restrictive physiology is present, the absence of calcification does not rule out constriction. Patients with constrictive pericarditis typically have a pericardial thickness greater than 4 mm (Figure 9-14). However, a thickened pericardial contour alone does not necessarily mean that constriction is present. The most reliable sign indicating constriction is the presence of pericardial adhesions. MRI can be used to determine if adhesions are present. Cine gradient echo movies with tag lines placed orthogonal to the pericardium would have to be prescribed. These black tag lines are placed early in systole and then move with the tissue during the cardiac cycle. Along normal pericardium the tag lines are expected to break because the two layers of pericardium can freely move with respect to each other. In constriction, adhesions limit the motion between the pericardial layers, and the tag lines do not break across the pericardium but have a stretched appearance (Figures 9-15, 9-16). Cine images may also show a paradoxical motion of the ventricular septum.
Because of the restriction to right ventricular filling, the right atrium, venae cavae, and hepatic veins are dilated. A pitfall in examining calcific pericarditis with MRI is that the calcified pericardium has a signal void (see Figure 9-13).
Congenital Absence of the Pericardium
Location of Defects
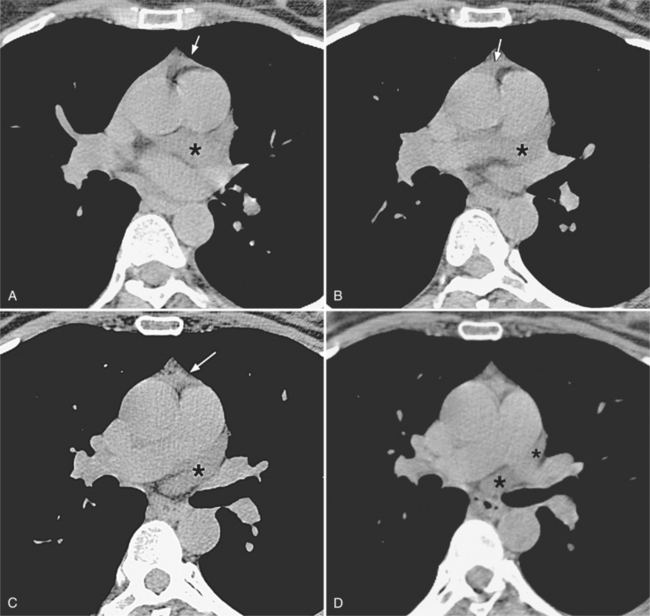
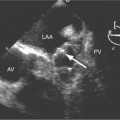
Congenital absence of the pericardium may involve all or part of the parietal pericardium. Most defects are partial and involve a defect over the left atrial appendage and adjacent pulmonary artery (Figure 9-17). Defects in the diaphragmatic part of the pericardium and partial defects over the right atrium and superior vena cava are much less common, and total absence is extremely rare (Figure 9-18). About 20% of patients with pericardial defects have associated heart and mediastinal abnormalities, including atrial septal defect, patent ductus arteriosus, tetralogy of Fallot, bronchogenic cysts, and pulmonary sequestration. Patients with partial pericardial defects are at risk for having part of the heart herniate through the defect, which could cause local strangulation of that part of the heart. In partial absence over the left side, the left atrial appendage may be strangulated.
Radiologic signs
Most of these defects can be identified on the plain chest film (see Figures 9-17, 9-18). Defects on the left side of the mediastinum rotate the heart in that direction, producing levocardia. The radiologic signs of absent pericardium include:
Pericardial Masses
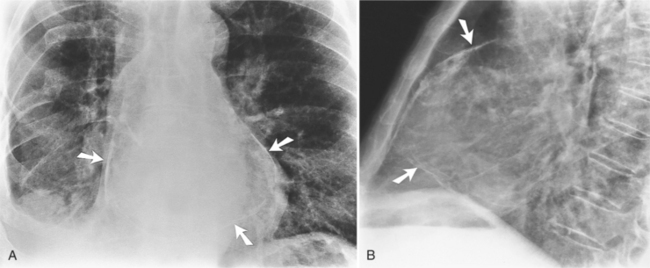
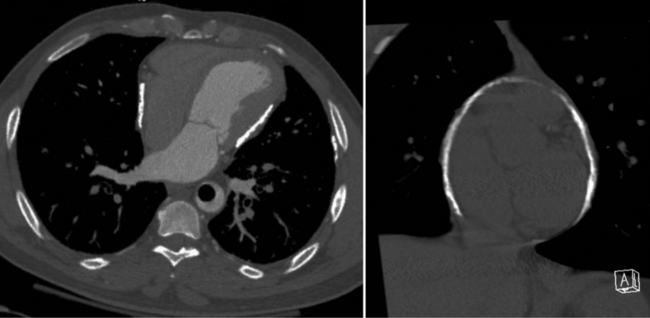
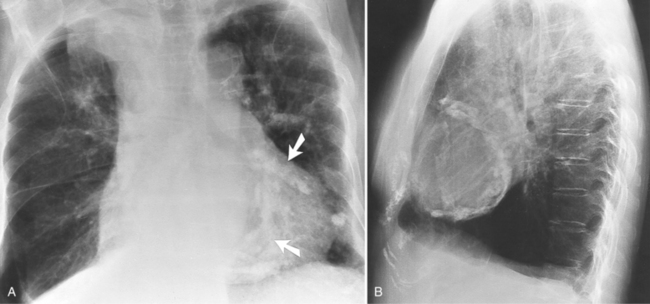
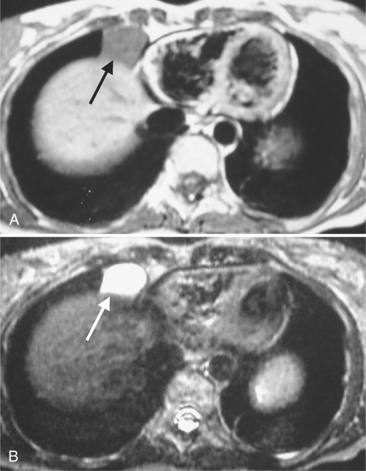
Focal masses in the pericardium may originate in the heart, in the pericardium, or in adjacent structures. Pericardial metastases are common (Figure 9-19). They are found in half of patients dying from breast or lung carcinoma. Primary pericardial masses are usually cysts or lipomas. Cysts in pericardial teratomas contain all three germ layers, whereas intrapericardial bronchogenic cysts contain two germ layers. Some 70% of cysts occur in the right cardiophrenic angle; the rest occur in the left cardiophrenic angle and in the anterior mediastinum (Figure 9-20).
Not all pericardial masses are tumors. Bronchogenic cysts develop in the neonate and are quite rare. Herniation of abdominal contents into the pericardium can occur through a partial absence of the diaphragm. Purulent pericarditis can progress to a pericardial abscess (see Figure 9-7).