CHAPTER 113 Peripheral Artery Disease
Peripheral arterial disease, most often a result of atherosclerosis, occurs in approximately 10% of the adult population and affects more than 10 million people in the United States alone.1 Most frequently, patients present with claudication. Less often, patients suffer from critical limb ischemia. Risk of illness or death from other cardiovascular causes in these patients with chronic limb ischemia is greatly increased, making peripheral arterial disease an important marker for atherosclerotic cardiovascular disease in general.2
ACUTE LIMB ISCHEMIA
Prevalence and Epidemiology
The incidence of acute limb ischemia is approximately 1.7/10,000 per year.3 Patients presenting with a pulseless extremity suffer amputation rates as high as 10% and mortality rates of 5% to 15%.4,5 These patients are characteristically at high risk for perioperative complication and are medically fragile (Table 113-1).
Etiology and Pathophysiology
Limb ischemia can result acutely from a variety of causes (Table 113-2). Sudden-onset acute pain is indicative of an embolic event; a history of chronic pain or claudication before the acute ischemic episode is suggestive of a thrombotic etiology. Graft occlusions are slightly more common than thromboses of native arteries. Thrombotic occlusions are approximately six times more common than embolic events.
TABLE 113-2 Causes of Acute Limb Ischemia
| Embolism (often to an arterial bifurcation) |
Bypass graft thrombosis has become the most frequent cause of acute lower extremity ischemia. Intimal hyperplasia and valvular hyperplasia are the most common causes of thrombosis in native conduit bypasses. In prosthetic grafts, acute thrombosis is most commonly due to kinking across joints or to the thrombogenicity of the graft material itself.6
Manifestations of Disease
Clinical Presentation
Because of its acuity and thus the absence of adequate preformed collateral arteries, arterial embolization is the classic situation resulting in acute arterial occlusion and acute limb ischemia. The anatomic distribution of arterial embolism is depicted in Figure 113-1. The embolus most frequently lodges at an arterial bifurcation.
In the patient with bilateral lower extremity ischemia and absent femoral pulses, the most common clinical scenario is saddle embolus to the aortic bifurcation. The patient will complain of sudden onset of bilateral buttock and lower extremity pain. Mottling of the lower extremities and lower abdomen, sometimes up to the umbilicus, will be evident on physical examination. The diagnosis in these patients is too frequently missed, probably because of the bilateral nature of the ischemic insult. Even in the setting of absent femoral pulses, patients are often evaluated for possible neurologic or neurosurgical problems, resulting in a delay of diagnosis and appropriate therapy. This disease can carry with it a poor prognosis, with a 27% mortality in one modern series.7
The most common site of clinically significant embolism in the lower extremity is the common femoral artery bifurcation.8 Patients with common femoral artery embolism will experience foot and calf pain. Finally, an embolus to the popliteal artery will result in absent pedal pulses and an ischemic foot.
Approximately 90% of the time, arterial emboli arise in the heart.9 Atrial fibrillation results in a dilated, noncontractile left atrial appendage, which predisposes to thrombus formation and—on spontaneous or therapeutic cardioversion—thromboembolism. Left ventricular thrombus may also form, for instance, adjacent to a noncontractile left ventricular segment after myocardial infarction, in a left ventricular aneurysm, or in the setting of dilated cardiomyopathy. Saddle emboli, which lodge at the aortic bifurcation and cause bilateral lower extremity ischemia, are most commonly the result of left ventricular thrombus. Thromboembolism from heart valves are less common today than in the past owing to the relative decrease in prevalence of rheumatic heart disease. Bacterial endocarditis can result in septic emboli that may cause both acute ischemia and infection of the distal vessel wall, which in turn results in mycotic aneurysm. Atheroemboli may arise from either the thoracic or the abdominal aorta. Finally, in some 5% of cases, the source of embolism is never identified.
Clinical Categorization of Acute Limb Ischemia
Since 1997, the Rutherford criteria have been used to grade the clinical severity of acute limb ischemia.10 These grades, as summarized in Table 113-3, are indicative both of whether emergent surgical intervention is indicated and of whether the limb is salvageable. Most commonly, category I represents an acute occlusion in a chronically narrowed artery, with well-formed collaterals. Category II represents a limb that is salvageable with immediate therapy or intervention. In the case of irreversible ischemia, category III, the patient will present with profound vascular and neurologic deficits; the limb may be in a state of rigor mortis and will require amputation.
Before imaging studies, in patients with acute limb ischemia, laboratory studies are indicated. In particular, and in anticipation of imaging studies with use of nephrotoxic contrast agents, a serum creatinine concentration should be obtained. If a hypercoagulable state is suspected, a hypercoagulable profile should be sent before institution of any anticoagulation. An electrocardiogram will aid in the diagnosis of atrial fibrillation and will provide some information as to the patient’s cardiac status. Assessment of the patient’s cardiac risk for general anesthesia by the Goldman index or other scale may be useful.11
Whereas some surgeons will elect to observe a transiently ischemic limb for signs of compartment syndrome postoperatively, in the setting of more than 6 hours of profound ischemia, many perform four-compartment fasciotomies prophylactically at the time of revascularization. If one elects to observe the patient’s leg, any increased pain, especially with passive plantar flexion of the foot, or loss of sensation in the first web space of the foot (sensory distribution of the deep peroneal nerve) should prompt reevaluation. Compartment pressures may be measured by a Stryker needle (Stryker Instruments, Kalamazoo, MI) or other device. Compartment pressure of greater than 30 mm Hg can result in tissue ischemia and necrosis. Patients demonstrating hypotension or shock, those requiring pressors, those with absent flow through the popliteal artery at presentation, and younger patients with greater muscle mass and fewer arterial collaterals are at increased risk for development of compartment syndrome. Four-compartment fasciotomy is usually performed through both a medial and a lateral incision (Fig. 113-2). The incisions are left open for subsequent delayed primary closure or skin grafting.
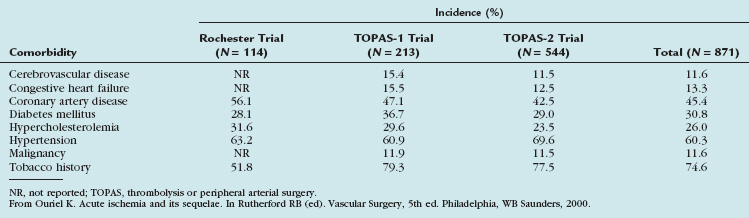
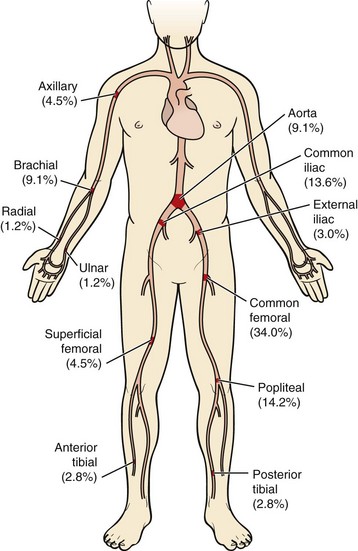
 FIGURE 113-1
FIGURE 113-1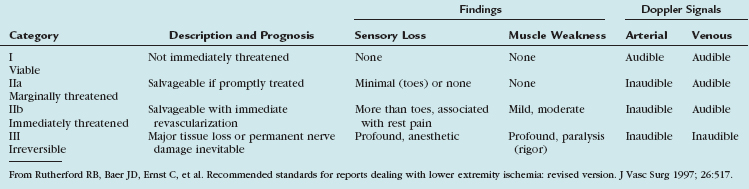
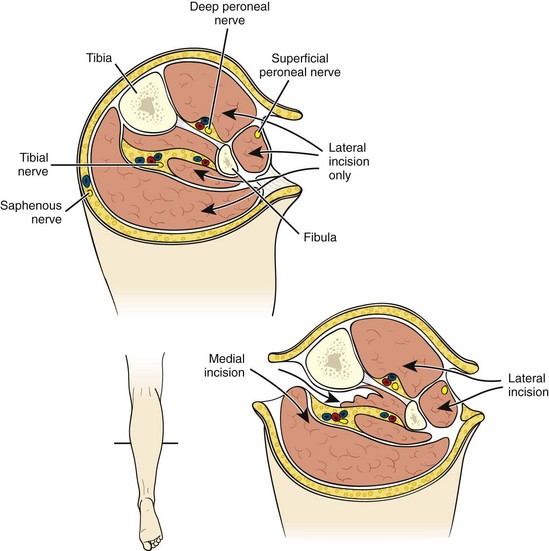
 FIGURE 113-2
FIGURE 113-2


