CHAPTER 116 Peripheral Magnetic Resonance Angiography
MR angiography (MRA) is a highly reliable technique that is widely used for imaging large and medium-sized arteries of the pelvis and lower extremities. In many hospitals worldwide, this technique has become an important adjunct to duplex ultrasonography (DUS) and x-ray catheter angiography, specifically intra-arterial digital subtraction angiography (IA DSA), in the workup of suspected peripheral artery disease (PAD). MRA, in many cases, is replacing diagnostic x-ray catheter angiography because it can provide similar diagnostic vascular road maps (Fig. 116-1) without the associated clinical concerns and risks related to invasive catheterization, ionizing radiation exposure, and use of iodinated contrast agents.
PAD (also called peripheral arterial occlusive disease or peripheral vascular occlusive disease) is almost invariably the result of advanced atherosclerosis of the pelvic and lower extremity arteries. With increasing age, atherosclerotic plaque develops in the walls of the lower extremity arteries, leading to luminal narrowing and often arterial occlusion. This progression of events results in a recognizable clinical constellation of signs and symptoms that typically begins as intermittent claudication and progresses to lower extremity pain at rest and even nonhealing skin ulceration. The diagnosis of PAD is typically initially made on the basis of a single measurement of the ankle-brachial index (ABI) below 0.9.1
PREVALENCE AND EPIDEMIOLOGY
Atherosclerotic PAD is an important health care problem in Western society, with an estimated prevalence of about 3% of the general population in those older than 50 years. The prevalence rises to about 15% to 20% of the general population in those older than 70 years.1
ETIOLOGY AND PATHOPHYSIOLOGY
In a minority of patients with intermittent claudication, the PAD will subsequently progress to critical ischemia, whereby even at rest the lower extremity arterial flow is insufficient to meet basal resting metabolic needs (i.e., oxygen and nutrient demand) of the lower extremity. Clinically, this is manifested by pain at rest and, in more severe cases, as nonhealing ulcers, cellulitis, and even gangrene. See Chapter 113 for an extensive discussion of the clinical aspects of PAD.
MANIFESTATIONS OF DISEASE
Clinical Presentation
The diagnosis of PAD is made on the basis of the typical history, physical examination (palpation of arterial pulsations), and measurement of the ABI. When a patient presents to the general practitioner or vascular surgeon with complaints of PAD, first-line treatment consists of modification of and/or treatment for atherosclerotic risk factors, such as smoking, hypertension, hypercholesterolemia, and the institution of (supervised) exercise training.2,3 Only when the patient’s complaints become too limiting to pursue regular activities will invasive interventional treatments be considered. For patients with intermittent claudication, the decision to intervene is largely dependent on relative criteria (patient and surgeon preference), but for patients with chronic critical ischemia, the need to intervene is more urgent because tissue perfusion does not meet basic metabolic demands, even at rest. Of 100 patients presenting with PAD, 5 eventually undergo percutaneous or surgical treatment.1 Although this is only a small minority of patients with PAD, the estimated annual number of percutaneous and surgical procedures performed for PAD in the United States alone was well over 200,000 in 2000, with sharp increases expected.4
Imaging Indications and Algorithm
Because the diagnosis of PAD is usually made from the typical history, physical examination, and ABI measurements, the need for imaging of the peripheral arteries only arises when a percutaneous or surgical intervention is considered. Imaging is needed to explore the extent of the disease process (e.g., number, location, and severity of atherosclerotic lesions) and to plan the correct approach for therapy.5
Traditionally, the standard of reference for imaging PAD has been x-ray catheter angiography, which initially had been through a translumbar aortic approach. In 1953, the transfemoral approach was developed by Seldinger, in which arterial access is gained through the superficial or common femoral artery.6 Having been refined and technically optimized, this is the procedure most widely used in state of the art angiography today, and is still considered the standard of reference. When combined with digital subtraction techniques, high-resolution projection arteriograms of the peripheral arterial circulation can be obtained in a routine fashion. However, substantial rates of local and systemic procedure-related complications have sparked the search for noninvasive alternatives to IA DSA.
Imaging Techniques and Findings
Ultrasound
DUS is widely used to determine the location, length, and severity of aortoiliac and femoropopliteal stenoses and obstructions. Duplex ultrasound was developed in the 1980s as an alternative to invasive angiography to avoid the inherent complications associated with the latter procedure. With DUS, the severity of a stenosis can be determined by using peak systolic velocity (PSV) measurements in arteries with reduced luminal diameter, PSV ratio at the site of stenosis and adjacent normal artery, end-diastolic velocity, and other less firmly established criteria. The sensitivity and specificity of DUS are generally moderate to high, ranging from 70% to 90%.7 However, a relatively recent meta-analysis,8 as well as a large prospective comparison study between CE MRA and DUS in 295 patients,9 has found that CE MRA is more sensitive and specific compared with DUS for the detection of PAD.
Computed Tomography
Recent advances in CT technology have enabled fast and robust CT angiography (CTA) of the peripheral vascular tree. Although there are fewer reports comparing CTA with conventional angiography for the detection of PAD as compared with CE MRA, it is widely believed that CTA is a valid and reliable method.10 The drawback of CTA is the enormous number of data sets that it generates—up to several thousand images per patient—and that heavily calcified arteries demand extensive user interaction to assess the underlying degree of stenosis adequately. In addition, the newest generation of multidetector row CT scanners is so fast that the contrast bolus may progress more slowly down the leg than the CT acquisition, leading to suboptimal opacification of the distal lower extremity arteries. For an in-depth discussion of CTA of the peripheral arteries, including these issues, see Chapter 115.
Magnetic Resonance Angiography
Although there are a variety of different MR angiography techniques, CE MRA is the most widely used method. Phase contrast (PC) and time of flight (TOF) MRA11 were the subjects of intense investigation about a decade ago, but the intrinsic drawbacks associated with these methods, such as long imaging times and their propensity to overestimate the degree and length of arterial stenoses, have led to the abandonment of these techniques in favor of CE MRA. The superiority of CE MRA over other MRA methods for peripheral artery imaging has been confirmed in several meta-analyses.12,13
Contrast-Enhanced Magnetic Resonance Angiography of the Peripheral Arteries
Synchronization of Three-Dimensional Contrast-Enhanced Magnetic Resonance Angiography Acquisition with Contrast Arrival
For successful CE MRA, care must be taken to synchronize peak arterial enhancement with image data acquisition, specifically acquisition of the central k-space data. The time of peak arterial enhancement is a function of many variables, the most important of which are injection rate and volume, amount and rate of saline flush,14 and cardiac output.15 Because the time of peak arterial enhancement can vary substantially among patients, the CE MRA examination needs to be tailored to the individual contrast arrival time. This is important for two main reasons: (1) to prevent “ringing” image artifacts and poor arterial opacification, which may occur if imaging is performed too early; and (2) to prevent suboptimal arterial enhancement and excessive venous and/or background enhancement, which occurs if imaging is performed too late.
Strategies to Optimize Vessel to Background Contrast
For multistation peripheral CE MRA (i.e., bolus chase CE MRA), the arterial T1 shortening associated with the sustained injection of a 0.1- to 0.3-mmol/kg dose of a standard (0.5 M) gadolinium-chelate contrast agent is generally insufficient to view the arteries preferentially over the extended FOVs over that of background tissue, especially in distal infrapopliteal arteries. The elimination of signal from background tissues, especially fat, because it has the shortest T1, is typically necessary for successful multistation peripheral CE MRA. The most commonly used technique to suppress background signal is image subtraction of nonenhanced mask three-dimensional MRA images from those of similarly acquired contrast-enhanced three-dimensional CE MRA. Although image subtraction decreases the signal-to-noise ratio by a factor of about 1.4 (v2 when the number of signals acquired is 1), vessel to background contrast improves to the extent that whole-volume MIPs become clinically useful, especially when using injection rates below 1.0 mL/sec.16 A disadvantage of using mask scans is that patients may move in between acquisition of the mask and contrast-enhanced parts of the scan, which can lead to subtraction misregistration artifacts. Subtraction misregistration artifacts may also occur if table positioning between the precontrast mask and postcontrast CE MRA is not accurate, on the order of 1 mm or less.
Because the T1 of fat is close to that of contrast-enhanced arterial blood, another way to suppress background tissue is by spectral saturation of signal from protons in fat. Although a fat saturation prepulse can be integrated into the three-dimensional CE MRA sequence, this takes a significant amount of time, which in turn must be offset by decreasing spatial resolution to achieve the same desired overall acquisition duration. Results of using fat saturation pulses are mixed and their use can, therefore, not be universally recommended.17,18
A dedicated peripheral vascular surface coil is mandatory for high-quality imaging of the pelvic and lower leg arteries. Image quality and anatomic coverage are vastly improved when compared with imaging without these dedicated lower extremity coils.19
Strategies to Decrease Venous Enhancement
Venous contamination is an important problem for CE MRA. This problem is particularly prevalent in patients with cellulitis or arteriovenous fistulas or malformations.20 Venous and background soft tissue contamination of arterial illustration are particularly prevalent in patients with diabetes mellitus.21 Diabetic patients, furthermore, are more likely to have limb-threatening ischemia and to require peripheral distal bypass surgery, making them prime candidates for preoperative peripheral artery imaging using CE MRA.
To avoid the limitations of imaging three consecutive stations, an alternative approach is to use two separate injections for peripheral CE MRA. In this approach, the distal lower legs (calves-feet or infrapopliteal region) are imaged during the first contrast medium bolus, and then a second contrast medium injection is administered to image the remaining two more proximal stations (the aortoiliac region and upper legs) using a two-station bolus chase CE MRA method. A benefit of this hybrid approach22
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree



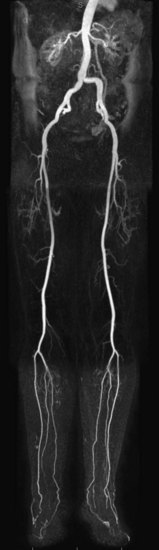
 FIGURE 116-1
FIGURE 116-1