The magnetic resonance neurography (MRN) examination is rapidly becoming a part of the diagnostic algorithm of patients with peripheral neuropathy; however, because of the technical demands and the lack of required reading skills, the examination is relatively underutilized and is currently limited to a few tertiary care centers. The radiologists with interest in peripheral nerve imaging should be able to perform and interpret this examination to exploit its potential for widespread use. This article outlines the systematic, stepwise approach to its interpretation and a brief discussion of the imaging pitfalls.
Key points
- •
Normal peripheral nerves demonstrate a size similar to the adjacent arteries, and the size and signal intensity are nearly symmetrical bilaterally.
- •
Systemic and polyneuropathy conditions may be incidentally discovered on magnetic resonance neurography; however, their diagnosis is primarily based on clinical and electrodiagnostic findings.
- •
In entrapment neuropathy, the signal, fascicular, and size abnormality of the peripheral nerve are maximum at and just proximal to the site of entrapment with distal gradual return to normalcy.
- •
In nerve injury, it is important to differentiate stretch injury from neuroma in continuity and nerve discontinuity because of the different treatment implications.
- •
The triple-B sign is a sign of severe peripheral neuropathy, which will require surgery, in many cases.
- •
Muscle denervation change usually shows diffuse involvement with a lack of associated perimuscular edema, hemorrhage, or nearby fascial involvement as opposed to myopathy/myositis cases, which usually involve multiple muscle compartments and are frequently associated with above findings.
- •
Two-dimensional imaging is most useful for the depiction of detailed nerve architecture and regional pathology.
- •
Three-dimensional imaging is useful to produce a longitudinal display of the nerves for discussing cases with referring physicians, depicting course deviations/focal changes in nerve caliber, and demonstrating the relationship to the adjacent space-occupying lesions for presurgical planning.
Introduction
Magnetic resonance neurography (MRN) is an exquisite technique for the visualization of peripheral nerve anatomy and lesions. Proper performance of MRN requires an understanding of nerve anatomy; a high-strength MR machine; good-quality gradient coils; and high-resolution as well as high-contrast, 2-dimensional (2D) and 3D, predominantly spin echo–type imaging techniques. For accurate MRN interpretation, it requires a detailed understanding of nerve anatomy and pathophysiology, normal and abnormal imaging appearances of the peripheral nerves on various imaging techniques, and a systematic approach to evaluate the spectrum of nerve lesions. This article outlines the MRN technical considerations, normal and abnormal peripheral nerve imaging appearances, current indications of MRN, and a systematic approach to diagnose these lesions.
Introduction
Magnetic resonance neurography (MRN) is an exquisite technique for the visualization of peripheral nerve anatomy and lesions. Proper performance of MRN requires an understanding of nerve anatomy; a high-strength MR machine; good-quality gradient coils; and high-resolution as well as high-contrast, 2-dimensional (2D) and 3D, predominantly spin echo–type imaging techniques. For accurate MRN interpretation, it requires a detailed understanding of nerve anatomy and pathophysiology, normal and abnormal imaging appearances of the peripheral nerves on various imaging techniques, and a systematic approach to evaluate the spectrum of nerve lesions. This article outlines the MRN technical considerations, normal and abnormal peripheral nerve imaging appearances, current indications of MRN, and a systematic approach to diagnose these lesions.
Nerve anatomy
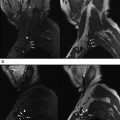
Peripheral nerves form a part of the neurovascular bundle, and their caliber is usually similar to an adjacent artery. The nerve size gradually decreases along its distal course with minimal variations near the ramifications and joints. Peripheral nerves encompass a unique organizational structure. The smallest unit is an axon enveloped by a layer of Schwann cells and connective tissue stroma and is referred to as the endoneurium . The axons are bathed by endoneurial fluid, which shows antegrade and, possibly, retrograde axoplasmic flow. The axons are bundled together to form a fascicle, which in turn is covered by another connective tissue layer called the perineurium . The fascicular bundles may range from 2 to 10 on imaging in commonly visualized peripheral nerves and may contain motor, sensory, or sympathetic fibers in various combinations. The final connective tissue layer, named the epineurium , covers all of the fascicles within the nerve. There are internal and external epineurium layers surrounding and enveloping the fascicles, respectively. The epineurium and perineurium are uniformly thin structures. On current high-resolution MRN techniques, the normal peripheral nerves (more than 2–3 mm) are easily distinguished from the surrounding vessels because of their unique fascicular appearance, nonbranching, relatively straight course in most parts, and lack of flow voids. The normal nerves are expected to show isointense signal intensity on T1-weighted (W) and T2-W MR images ( Fig. 1 ). Minimal T2 hyperintense signal alteration is commonly observed on the inversion recovery images because of the increased sensitivity to endoneurial fluid, better fat suppression, and increased dynamic range of contrast. The hyperintensity is particularly prominent on 3D inversion recovery images; however, it should be symmetric from side to side. Using good surface coils, the fascicles are consistently depicted in normal nerves that are 3 mm or larger. The pencil-thin epineurial layer can also be seen forming the outer covering of the nerve in larger nerves. The perineurial and epineurial layers are relatively imperceptible, unless abnormally thickened ( Fig. 2 ). Normal and abnormal nerve MRN imaging appearances are outlined in Table 1 .
| Nerve | Normal | Abnormal |
|---|---|---|
| Size | Similar to adjacent artery Gradually decreases distally | Focal or diffuse enlargement Larger than the adjacent artery |
| Signal intensity | T1W & T2W: isointense to skeletal muscle STIR/fat suppressed T2W: isointense to minimally hyperintense 3D TSE (turbo spin echo): uniform and symmetric hyperintensity | T2 hyperintensity (becoming similar to adjacent veins) Asymmetric hyperintensity on 3D TSE |
| Fascicular pattern | T1W & T2W: present and uniform | Single or multiple fascicles enlargement/disruption Loss of fascicular pattern |
| Course | Smooth without focal deviations | Focal or diffuse deviations Discontinuity |
| Enhancement | Absent (except in areas of deficient blood-nerve barrier, such as dorsal nerve root ganglion) | In tumors & infections (disruption of the blood-nerve barrier) |
| Perineural fat | Clean fat planes | Perineural strand like T1 & T2 hypointensities/nerve encasement |
| Diffusion tensor imaging, tracts | Normal tracts | Abnormal tracts, disrupted or displaced |
| Diffusion tensor imaging, quantitative | Normal fractional anisotropy values (>0.4–0.5) | Abnormal fractional anisotropy values (<0.4–0.5) |
| Diffusion tensor imaging, qualitative | Symmetric brightness of nerves on tensor images | Asymmetrical hyperintensity of the neuropathic nerve |
Nerve pathophysiology
Peripheral nerves may be involved in a variety of pathologies, which are broadly divided as systemic and local conditions. The systemic pathologies include vasculitis; toxic and metabolic diseases, such as diabetes mellitus (DM), amyloidosis, and hyperlipidemia; hereditary conditions, such as Charcot-Marie-Tooth (CMT) disease; idiopathic conditions, such as multifocal motor neuropathy (MMN); acute or chronic demyelinating conditions, such as chronic demyelinating polyneuropathy (CIDP); and neurocutaneous syndromes, such as neurofibromatosis (NF) and schwannomatosis (SW). The local conditions include compressive or adhesive neuropathies, such as tunnel syndromes; nerve injury; infection; peripheral nerve sheath tumor (PNST) and perineural tumor; or functional or traction neuropathy from activities, such as repeated typing, bad footwear, ankle instability, and functional compartment syndromes.
In systematic conditions, the spinal cord, dorsal nerve root ganglion, axons, or the surrounding myelin sheath are affected in various combinations. The diagnosis is generally clinical and biochemical with frequently multi-compartment or multifocal nerve abnormality observed in the aforementioned pathologies. Electrodiagnostic studies, such as electromyography and nerve conduction study, are currently the diagnostic technique of choice to evaluate these lesions. MRN is uncommonly used to evaluate these subjects and might be used in cases when there are worsening focal/regional symptoms to exclude a superimposed entrapment or a mass lesion. Sometimes, the neuropathy related to the systemic conditions may be incidentally observed in studies performed for suspected nerve entrapment or injury, especially diabetic neuropathy, which is not uncommonly encountered during joint/extremity imaging. Therefore, the reader should be aware of neuropathy findings related to such conditions. Although hereditary neuropathy leads to symmetric disease with marked nerve thickening and hyperintensity, especially in CMT type IA (demyelinating type) ( Fig. 3 ), acquired conditions commonly lead to asymmetrical nerve thickening or hyperintensity. Diabetic neuropathy is seen as abnormal T2 hyperintensity with or without prominent fascicles, symmetric and multi-nerve involvement, nerve enlargement, and internal fat proliferation with chronicity.
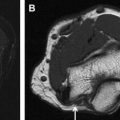
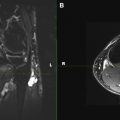
Most commonly imaged local conditions can be subdivided into nerve entrapment or nerve injury. Nerve entrapment caused by increased pressure within a tunnel (confined space through which the nerve traverses) or from a space-occupying lesion leads to the blockade of axoplasmic flow, venous congestion, hyperemia, epineurial edema at and proximal to the site of entrapment, as well as wallerian degeneration distally. The larger myelinated axons situated in the outer portions of the nerve are the earliest and most affected, supporting the theory that the primary mechanism of injury is a shear force, which is followed by ischemia. These pathologic abnormalities lead to hyperintense nerve signal change on T2-W MR images, with signal intensity approaching approximately 90%, similar to adjacent vessels confirming neuropathy. The signal abnormality is also maximum at and just proximal to the site of entrapment with distal fading/resolution to normal signal intensity. Associated nerve enlargement and fascicular abnormality becomes apparent with increasing severity of neuropathy (see Fig. 1 ). Both proximal and distal nerve enlargement may be seen with entrapment on MRN. The proximal enlargement is much more common and pronounced than the distal enlargement, and the latter is usually observed in more severe cases of entrapment. This finding also supports the theory of bidirectional axoplasmic flow. With chronic entrapment, the epineurial edema incites fibrotic changes causing further nerve entrapment, axonal degeneration, and myelin loss. Thus, mild entrapment neuropathy is identified as mild-moderate hyperintensity or mild nerve flattening. Moderate entrapment shows one or a combination of imaging findings of moderate to severe hyperintensity, fascicular abnormality, and proximal nerve enlargement with distal flattening; whereas severe neuropathy is seen as the aforementioned findings as well as abrupt changes in the nerve signal intensity from bright-black-bright (triple-B sign) along the length of the nerve. Signal intensity on diffusion tensor imaging (DTI) also increases nearly proportionate to the degree of neuropathy; however, more research is needed to establish the value of DTI in nerve imaging ( Fig. 4 ). Space-occupying lesions affecting the nerves can be broadly characterized into intraneural and perineurial lesions. Intraneural lesions lead to nerve enlargement and/or fascicular involvement ( Fig. 5 ), whereas perineurial lesions cause nerve encasement or displacement.
Nerve injuries have been traditionally classified by the Seddon and Sunderland grading systems based on injury to various layers of the nerve. However, for practical management planning and while interpreting MRN examinations, nerve injuries can be conveniently divided into the following: (1) stretch injury (which could be mild- observed as abnormally hyperintense nerve, otherwise in continuity and with nearly similar size to the adjacent vessels or contralateral nerves; or Moderate- observed as moderate diffuse nerve enlargement more than the size of the adjacent vessels or contralateral nerves because of axonal degeneration and the expansion of fluid space within the nerve); (2) high-grade nerve injury with nerve in continuity or neuroma in continuity (abrupt change in nerve caliber with focal nerve enlargement and effaced fascicular appearance) ( Fig. 6 ); and (3) nerve root avulsion or nerve transection (complete discontinuity) with end bulb neuroma formation. Stretch injuries usually require conservative management and rehabilitation. Sometimes, neurolysis or nerve decompression may be attempted depending on the clinical situation. Surgery is commonly performed in high-grade nerve injury, painful neuroma in continuity, nerve avulsion, and transection because the functional return is often incomplete or none in these injuries. An important imaging feature of alternating signal alteration of bright-black-bright (triple-B sign) along the length of the nerve is an indicator of severe focal neuropathy that can be seen with nerve transection, very severe entrapment, loss of nerve coaptation, dehiscence/degeneration of nerve graft, or freeze injury related to cryoablation ( Fig. 7 ).