Peritoneal Fluid Collections
Etiology
Ascites is the abnormal accumulation of fluid in the peritoneal cavity. There are numerous causes of ascites, including congenital, infective, inflammatory, and neoplastic diseases. In the United States the most common causes are liver disease and malignancy. In many parts of the world, tuberculosis is an important cause. The main causes of ascites and their frequency in the United States are listed in Table 81-1 .
| Cause | Percentage |
|---|---|
| Cirrhosis | 81 |
| Malignancy | 10 |
| Heart failure | 3 |
| Tuberculosis | 2 |
| Dialysis | 1 |
| Pancreatic disease | 1 |
| Others | 2 |
Prevalence and Epidemiology
The term ascites does not specify the type of fluid accumulated within the peritoneal cavity. Ascites may be further classified as in Table 81-2 into infected, chylous, hemorrhagic, and neoplastic fluid.
| Type | Characteristics |
|---|---|
| Infected | Attenuation of fluid collection ≥20 HU Loculated Rim enhancing Air ± |
| Chylous (discussed in text) | Fat/fluid level Fluid/fluid level Density equal to that of water |
| Hemorrhagic (discussed in text) | High density fluid or clotted blood Density ≥50 (Density may vary as blood may be clotted or lysed) Retroperitoneal spread ± |
| Neoplastic | Solid mass or metastatic nodules Abundant fluid May or may not be loculated |
Specific Types
Chylous Ascites
Chylous ascites is a milky fluid that is rich in triglycerides secondary to leakage of lymph into the peritoneal cavity. In the United States the most common cause of chylous ascites is malignancy, of which lymphoma accounts for 30% to 50% of cases. Other associated neoplasms include breast, esophageal, pancreatic, colon, renal, testicular, ovarian, and prostate cancer, as well as lymphangiomyomatosis, a more common cause in children. Approximately 0.5% to 1% of cirrhotic patients with ascites have chylous instead of serous fluid.
Trauma, surgery, or radiotherapy to the abdomen may damage lymphatic channels and lead to chylous ascites. Worldwide, infectious causes such as tuberculosis and filariasis (parasitic infection caused by Wuchereria bancrofti ) are more common than neoplastic causes.
Paracentesis typically shows a cloudy milky aspirate. Triglyceride content of more than 0.1 g/L is diagnostic of chylous ascites. When the cause of chylous ascites is unclear, computed tomography (CT) of the abdomen and pelvis may be useful to evaluate for lymphadenopathy.
Hemorrhagic Ascites
Persons with hemorrhagic ascites have a red blood cell count greater than 50,000/mm 3 . The normal red blood cell count of peritoneal fluid is less than 1000/mm 3 . There are several causes of hemorrhagic ascites. Bloody ascites occurs in approximately 5% of patients with cirrhosis. In such patients, hemoperitoneum may occur spontaneously or after a traumatic paracentesis. In the more common setting of traumatic paracentesis, the ascitic fluid will clot, in contrast to nontraumatic bloody ascites, in which the red cells are lysed and the fluid does not clot on standing. The presence of nontraumatic bloody ascites in a cirrhotic patient raises concern for an underlying malignancy such as hepatocellular carcinoma. Approximately 20% of ascitic fluid aspirations in patients with malignant ascites are bloody.
Trauma is clearly an important cause of hemoperitoneum (to be discussed). Other less common causes of hemorrhagic ascites are peritoneal dialysis, tuberculosis, rupture of vascular tumor such as hepatic adenoma, sarcoidosis, and vasculitis such as Henoch-Schönlein purpura.
Pathophysiology
There are three theories that explain the genesis of ascites. The diminished effective volume theory and overflow theory differ in whether abnormal renal sodium retention precedes or follows the accumulation of ascites. The peripheral arterial vasodilation theory combines aspects of both the volume and overflow theories and is the most widely accepted.
Imaging
Radiography
Plain films are insensitive to ascites until at least 500 mL of fluid has accumulated. Indirect and nonspecific signs are abdominal haziness, bulging of the flanks, indistinct psoas margin, and increased separation of bowel loops. More specific signs include separation of lateral liver contour from the thoracoabdominal wall (Hellmer’s sign), separation of the ascending and descending colon from the properitoneal fat line, and symmetric density on either side of the urinary bladder (the “Mickey Mouse” sign).
Ultrasonography
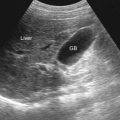
Ultrasonography detects as little as 10 mL of fluid. It is of help in assessing patency and flow pattern of portal or hepatic veins and in guiding paracentesis ( Table 81-3 ). Peritoneal fluid is seen in the pelvic cul-de-sac in normal females in all phases of the menstrual cycle. Features that differentiate simple from complicated ascites on imaging studies are shown in Table 81-4 and illustrated in Figure 81-1 . The findings of simple ascites does not exclude infection or tumor. Gallbladder wall thickening is seen in 82% of cases of benign ascites, whereas only 5% of malignant ascites show this finding. Ascites may cause artifacts as a result of reflection of the ultrasonic sound waves at the liver/fluid interface. Pericolonic epiploic appendages may simulate peritoneal metastases.
| Modality | Accuracy | Limitations | Pitfalls |
|---|---|---|---|
| Ultrasonography | Can detect as little as 10 mL of ascites Can assess portal and hepatic venous flow Portable | Overlying bowel gas, patient body habitus, limited evaluation of peritoneal masses | Artifacts from reflection of fluid-solid interfaces. Epiploic appendages can be mistaken for peritoneal metastases. |
| CT | The best single test for determining site and nonliver cause of ascites | Cannot detect low-density soft tissue in ascitic fluid Low sensitivity for serosal bowel metastases | A collection with considerable amount of solid tissue (e.g., in setting of pancreatic necrosis) may appear as simple fluid collection. |
| MRI | Good overview of ascites | Ill patients may not tolerate long scan. | Standing wave artifact on 3.0-T MRI. |
| Imaging Type | Simple Ascites * | Complicated Ascites † |
|---|---|---|
| Ultrasonography (see Figure 81-1 ) | Anechoic Acoustic enhancement Fills the space between organs and bowel without mass effect Mobile with patient’s position change Compresses with transducer pressure Thickened gallbladder Diffuse smooth thickening of small bowel without nodularity | Internal echoes Septa: Multiple septa suggest tuberculosis or pseudomyxoma Fluid displaces bowel and solid organs Scalloping of solid organ surface (liver, spleen) suggests pseudomyxoma Loops of bowel matted together Fluid in the lesser sac Loculated fluid collections Lack of thickening of gallbladder Peritoneal solid or cystic masses suggest malignant disease or less likely tuberculosis |
| CT | Uniform attenuation of 0 to 20 HU Bowel floats freely in midabdomen Ascites that does not extend onto the lesser sac | Loculated collections Peritoneal thickening or abnormal enhancement Peritoneal, omental masses or nodularity Attenuation >20 HU or variable attenuation Enhancement of peritoneal fluid on delayed phases |
| MRI | Fluid that is uniformly low signal on T1 weighting and very high signal on T2 weighting | Higher signal fluid on T1 weighting Debris within fluid Loculated fluid |
* Simple ascites denotes transudative fluid as seen in liver disease and cardiac failure.
† Complex ascites indicates the presence of infection, inflammation, or neoplasm. Hemorrhagic ascites is dealt with separately in the text.

Hemoperitoneum may have differing appearances depending on transducer frequency and duration of hemorrhage. At 2- to 3-MHz acute hemorrhage is anechoic with increased through-transmission. With increasing frequency of transducer, the hemorrhage appears echogenic. After the first 4 days, with hematoma lysis, internal echoes either fill the collection or layer dependently. With time, hematoma becomes an anechoic seroma.
Computed Tomography
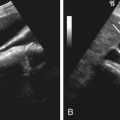
It is difficult to characterize the underlying cause of ascites based on CT attenuation of ascitic fluid. Simple ascites has a density of 0 to 30 Hounsfield units (HU). However, CT cannot differentiate among bile, urine, and serous fluid within the ascites. Chylous ascites has density less than 0 HU ( Figure 81-2 ). Hemoperitoneum usually has a density higher than 30 HU (see later). CT is not sensitive to the presence of semisolid material within the ascitic fluid ( Figure 81-3 ). This is particularly true with severe acute pancreatitis, in which peripancreatic necrosis is difficult to differentiate from inflammatory fluid based on CT density. Magnetic resonance imaging (MRI) is superior in demonstrating solid material within the ascitic fluid (see Figure 81-3 ). However, CT is better at showing small amounts of gas within collections.


The sensitivity of CT for detecting early subperitoneal metastases is low. In a study of 24 patients, the sensitivity of CT for peritoneal metastases less than 1 cm was 20% to 33% compared with 85% to 90% of gadolinium-enhanced MRI. Despite these potential pitfalls, in most institutions, CT is the principal imaging technique for detecting peritoneal disease and assessing the cause of unexplained ascites.
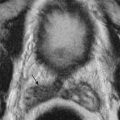
The CT appearance of blood in the peritoneal cavity depends on its duration. Owing to its high protein content, unclotted peritoneal blood usually has a measured attenuation of 30 to 45 HU; the attenuation may be lower in an anemic patient. Blood that has clotted has an attenuation of 45 to 70 HU. A sentinel clot is blood clotted adjacent to the bleeding site ( Figure 81-4 ). Narrow CT windows may be required to detect this clot. Layering of dense blood in the presence of ascites is often seen ( Figure 81-5 ). Active arterial extravasation shows higher density of 80 to 200 HU on a contrast-enhanced study. The presence of active extravasation usually mandates invasive therapy, whereas most other cases of hemoperitoneum may be treated conservatively.


Magnetic Resonance Imaging
The reader is referred to the section on peritonitis for a discussion of MRI techniques for the assessment of peritoneal disease.
Acute blood that contains deoxyhemoglobin is isointense and hypointense on T1- and T2- weighted sequences, respectively. Subacute blood containing methemoglobin is hyperintense on T1-weighted images. Initially, methemoglobin is intracellular and appears hypointense on T2-weighted sequences; as the red cells lyse, the signal is increased. Hemorrhage that is several days old is hypointense on all sequences owing to the presence of hemosiderin, which also causes susceptibility artifacts on gradient echo sequences.
Specific Lesions
Mesenteric Trauma
Bowel and mesenteric injuries are found in 5% of patients undergoing laparotomy for blunt abdominal trauma. Diagnostic peritoneal lavage has a sensitivity exceeding 90% for the detection of hemoperitoneum but does not accurately delineate which organ has been injured, does not detect retroperitoneal injury, and makes subsequent CT assessment difficult. CT is the best noninvasive method of detecting bowel and mesenteric injury with specificity of more than 95% and sensitivity of 70% to 95%. Table 81-5 gives the CT findings of bowel and mesenteric trauma ( Figures 81-4 and 81-6 ). Of note, bowel and mesenteric injuries often coexist.
| Injury | Specific CT Signs | Less-Specific CT Signs |
|---|---|---|
| Mesenteric injury (see Figures 81-4 and 81-6 ) | Mesenteric arterial extravasation Irregularity of mesenteric vessels with surrounding soft tissue density Abrupt termination of mesenteric vessel Hemoperitoneum without solid organ injury | Mesenteric fat haziness (sensitive but not specific) Mesenteric hematoma Ascites or retroperitoneal fluid collection |
| Bowel injury | Bowel wall discontinuity Extravasation of orally introduced contrast agent Pneumoperitoneum (without penetrating injury or prior diagnostic lavage) | Bowel wall thickening Abnormal bowel wall enhancement |

Abdominal Compartment Syndrome
Compartment syndrome is well known in the extremities, where increased pressure within a closed fascial space depresses capillary perfusion pressure to a level that cannot maintain tissue viability. The effects of elevated intra-abdominal pressure are less well recognized. Normally, intra-abdominal pressure is approximately 5 mm Hg. The intra-abdominal pressure may increase with acute and substantial accumulation of fluid within the abdomen ( Table 81-6 ). Abdominal compartment syndrome (ACS) is defined as intra-abdominal pressure of at least 20 mm Hg, with dysfunction of at least one thoracoabdominal organ, usually pulmonary or renal insufficiency. In contrast, a more gradual rise in abdominal pressure, such as in normal pregnancy or in ascitic accumulation in liver disease or ovarian cancer, does not normally result in ACS. Laparoscopy with pneumoperitoneum may cause elevated intra-abdominal pressure, but the effect is transient and not to the degree required to cause ACS. The definitive test for ACS is indirect measurement of abdominal pressure using a transurethral probe inserted in the urinary bladder. CT signs (see Figures 81-5 and 81-7 ; Box 81-1 ) are neither sensitive nor specific for ACS. However, when a combination of these findings is present in the appropriate clinical setting or if the signs are seen to worsen on sequential imaging studies, the radiologist should raise the possibility of ACS. As in other compartment syndromes, the definitive treatment of ACS is decompressive surgery.
| Potential Causes | Specific Conditions Increasing Risk |
|---|---|
| Trauma | Grade V liver injury Hemoperitoneum Penetrating trauma |
| Abdominal surgery | Surgery in obese patients Liver transplantation Repair of large incisional hernia |
| Pancreatitis | Hemorrhagic pancreatitis Large amount of pancreatic ascites |
| Massive fluid resuscitation | More than 5 L within 24 hr |

- •
Elevated diaphragm
- •
Rounded configuration of abdominal wall (anteroposterior-to-lateral girth ratio >0.8)
- •
Rapid increase in ascites (serial scans)
- •
Hemoperitoneum
- •
Flattened inferior vena cava
- •
Flattened renal veins
- •
Mosaic liver perfusion
- •
Increased bowel enhancement
- •
Increased gastric wall enhancement
- •
Gastric distention
- •
Reduced diastolic flow in portal, hepatic, or renal veins on ultrasonography
These computed tomography signs are neither sensitive nor specific. Alternative diagnoses such as hypotension, shock, bowel injury, and multiple-organ failure in severe pancreatitis may mimic the findings of abdominal compartment syndrome. Awareness of the clinical situation is required for raising the diagnosis—for example, most patients with abdominal compartment syndrome have normal blood pressure.
Analysis of Ascitic Fluid
The macroscopic appearance may be helpful in determining the cause. Uncomplicated ascitic fluid is usually a pale shade of yellow and is clear. Cloudy ascites may be seen in patients with liver disease owing to the presence of neutrophils, but this finding is not specific for infection. Chylous ascites typically has a milky appearance (see later). Malignant tumors or traumatic taps may produce bloody ascites. Dark-brown fluid may indicate the presence of bile.
Ascites was previously divided into either an exudate (>25 g/L of total protein content) or transudate (<25 g/L of protein). However, the serum ascites-albumin gradient has been found to be more helpful than the exudate-transudate concept in assessing the cause of ascites. The gradient is obtained by subtracting the ascitic albumin level from the serum level. A gradient of more than 1.1 g/dL indicates portal hypertension with a degree of accuracy purported to be 97%.
Treatment
Paracentesis
The mainstay of treatment of ascites, after medical therapy such as regulation of salt intake and diuretic therapy, is paracentesis. Many clinicians avoid performing paracentesis because of coagulopathy and fear of hemorrhagic complications. The number of patients who require red cell transfusion for paracentesis-related bleeding is less than 1%. The American Association of Liver Diseases does not recommend prophylactic fresh frozen plasma or platelet transfusion before paracentesis.
Before needle puncture, it is customary to use Doppler ultrasonography to check that there is no abdominal wall varix at the site of needle entry and an image of the site of puncture is obtained.
Other Treatment Options
Patients with ascites requiring repeated paracentesis should be considered for a transjugular intrahepatic portosystemic shunt (TIPS). If liver function is poor, patients should also be considered for liver transplantation. Peritoneovenous shunts (LeVeen or Denver) or surgical portosystemic shunts have very limited indications, even in those with refractory ascites.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree