PET-CT and SPECT-CT in Prostate Cancer
Daniela B. Husarik
Thomas F. Hany
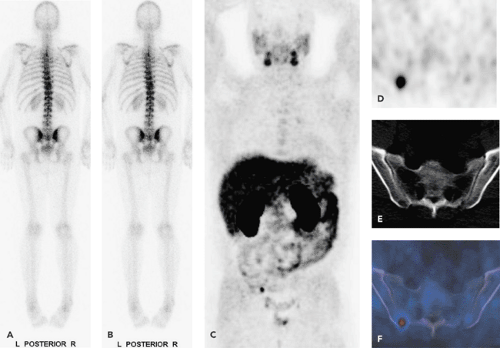
Positron emission tomography (PET) using [11C]- and [18F]-labeled choline analogs has shown promising initial results for prostate cancer staging and restaging. The role of choline-PET seems to be limited to N and M staging, because differentiation of benign prostatic hypertrophy from cancerous prostate lesions at initial staging is not possible with any choline PET-radiotracer analog. In patients with recurrent prostate cancer, PET with choline analogs is a promising imaging modality to detect local recurrence and lymph node metastases. Because of intestinal choline uptake and the close anatomic association with bowel, differentiation of tumor from normal choline uptake can be difficult. We therefore feel that the use of integrated PET/computed tomography (CT) in choline-PET examinations is a must (see Fig. 49.1).
Fluorodeoxyglucose (FDG)-PET has very limited value in prostate cancer; as with the well-differentiated and relatively slowly growing cancers, no FDG uptake is observed. Only when the cancer becomes androgen independent do the tumor manifestations become FDG avid.
Introduction
Prostate cancer is the leading cancer diagnosed and the second leading cause of cancer death in men. Sixty percent of all newly diagnosed prostate cancers and approximately 80% of all deaths from this cause occur in men aged 70 years and older. However, mortality rates are much lower than the incidence rates because survival is generally quite high. The causes of prostate cancer are not known.
Only insufficient evidence is present to establish whether screening by digital rectal examination or serum prostate-specific antigen (PSA) results in a decrease in mortality from prostate cancer. Diagnostic imaging includes ultrasound, computed tomography (CT), and magnetic resonance imaging (MRI) for local staging and bone scanning for detection of osseous metastases. As with most cancer types, adequate therapy is stage dependent. Whenever the prostate cancer is confined to the organ without evidence of local or distant metastases, radical prostatectomy, external-beam irradiation, and interstitial implantation of radioisotopes are used with similar therapeutic effects (1,2).
Nuclear imaging includes, in addition to bone scanning, some more specific agents, which have been evaluated, but the major impact may be coming from positron emission tomography (PET). Fluorodeoxyglucose (FDG)-PET has been used in several studies, but showed only slightly increased FDG uptake (see Fig. 49.2). Studies have shown that androgen-independent prostate cancer is positive in FDG-PET (3). Correspondingly, for the delineation of the primary tumor, the detection of metastases, or for the detection of recurrent disease after radical prostatectomy, FDG-PET provides questionable diagnostic benefits (4). Recently, other tracers such as carbon 11 choline and the choline analog fluorocholine (FCH) or [11C]methionine have shown encouraging results in the detection of local and distant metastases (5,6,7).
Non-PET Radiopharmaceuticals in Prostate Imaging
Bone scanning with Tc-labeled phosphonates is still an important staging method in the staging and follow-up
of patients with suspected or proven bone metastases of prostate cancer. The use of integrated single-photon emission computed tomography (SPECT)/CT in the detection of bone metastases in general is discussed in Chapter 52.
of patients with suspected or proven bone metastases of prostate cancer. The use of integrated single-photon emission computed tomography (SPECT)/CT in the detection of bone metastases in general is discussed in Chapter 52.
ProstaScint (111indium-labeled capromab pendetide) is a type II membrane glycoprotein strongly associated with prostate cancer and labeled with111indium used for planar and SPECT imaging in prostate cancer patients. Initial results reported encouraging results in the detection of local recurrence after therapy (8). However, during further investigations these results have been questioned, especially regarding extra-prostatic disease (9). ProstaScint is also being applied for evaluating patients with biochemical failure and in trying to discern metastasis before definitive therapy.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree