Imaging plays an integral role in the clinical care of patients with breast cancer. This review article focuses on the use of PET imaging for breast cancer, highlighting the clinical indications and limitations of 2-deoxy-2-[ 18 F]fluoro- d -glucose (FDG) PET/CT, the potential use of PET/MRI, and 16α-[ 18 F]fluoroestradiol (FES), a newly approved radiopharmaceutical for estrogen receptor imaging.
Key points
- •
Clinical indications of FDG PET/CT for breast cancer include initial systemic staging, suspected recurrence, and treatment response assessment.
- •
FDG PET/CT is not recommended for primary breast cancer detection, distinguishing benign from malignant breast lesions, or local tumor staging for newly diagnosed breast cancer.
- •
FDG PET/MRI has less radiation dose and may have superior performance for detecting hepatic and osseous metastases than FDG PET/CT.
- •
FES has recently obtained FDA approval for clinical use with PET imaging to detect ER-positive lesions in patients with recurrent or metastatic breast cancer.
Introduction
Breast cancer is the most common malignancy diagnosed in women and is the leading cause of cancer-related deaths for women worldwide. In 2020, approximately 276,480 women will be diagnosed with invasive breast cancer and 42,170 women will die from breast cancer in the United States. Breast cancer can also occur in men, although much less often than in women.
Breast cancer is a heterogeneous disease with several histologic and molecular subtypes associated with distinct prognoses and therapeutic options. The most common invasive histologic subtypes are invasive ductal carcinoma and invasive lobular carcinoma. Molecular subtypes of invasive breast cancer are classified by the expression of tumor biomarkers including estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor-2 (HER2). Hormone receptor positive (ER and/or PR positive), HER2-negative breast cancer is the most common molecular subtype (73%).
Imaging with mammography, ultrasound, magnetic resonance imaging (MRI), computed tomography (CT), and bone scintigraphy plays an integral part in the detection, staging, and monitoring of breast cancer. Positron emission tomography (PET) has also demonstrated clinical utility for breast cancer. This article reviews the use of PET imaging for patients with breast cancer, highlighting the clinical indications and limitations of 2-deoxy-2-[ 18 F]fluoro- d -glucose (FDG) PET/CT, the potential use of PET/MRI, and 16α-[ 18 F]fluoroestradiol (FES), a newly approved radiopharmaceutical for estrogen receptor imaging.
Clinical indications for FDG PET/CT
The Society of Nuclear Medicine and Molecular Imaging (SNMMI) and the European Association of Nuclear Medicine (EANM) have published procedure guidelines for oncologic imaging with FDG PET/CT. , These guidelines detail the clinical indications, performance, interpretation, and reporting of FDG PET/CT examinations. FDG PET/CT can be used for systemic staging of breast cancer, evaluation of suspected disease recurrence, and assessment of treatment response.
Initial Staging
Staging provides prognostic information regarding survival outcomes and is used to guide local, regional, and systemic therapy decisions. Breast cancer staging follows the American Joint Committee on Cancer (AJCC) TNM classification system. The anatomic stage includes the primary tumor size (T stage), regional lymph node status (N stage), and distant metastasis (M stage). Physical examination and conventional breast imaging (mammography, ultrasound, and MRI, if performed) are used to determine T and N clinical anatomic staging. Pathologic anatomic staging is based on surgical specimens from lumpectomy or mastectomy for T staging and sentinel lymph node biopsy or full axillary dissection for axillary N staging.
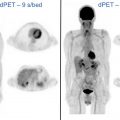
Approximately, 6% of all patients with newly diagnosed breast cancer have distant metastatic disease (M1; stage IV) at presentation. The most common sites are bone, lung, brain, liver, and distant lymph nodes. The use of imaging for the evaluation of distant sites of disease depends on the clinical stage and the presence of symptoms or laboratory abnormalities. For asymptomatic patients with early-stage disease (clinical stage 0-IIB), systemic imaging is not recommended by the National Comprehensive Cancer Network (NCCN) and the American College of Radiology (ACR). , Systemic imaging is indicated for patients with suspicious symptoms or laboratory abnormalities and for those with stage III, locally advanced and inflammatory breast cancers. Imaging options include CT, MRI, bone scintigraphy, and FDG PET/CT ( Figs. 1 and 2 ). Discovery of unsuspected distant metastases during initial staging is significant because clinical management then shifts from curative to palliative intent.

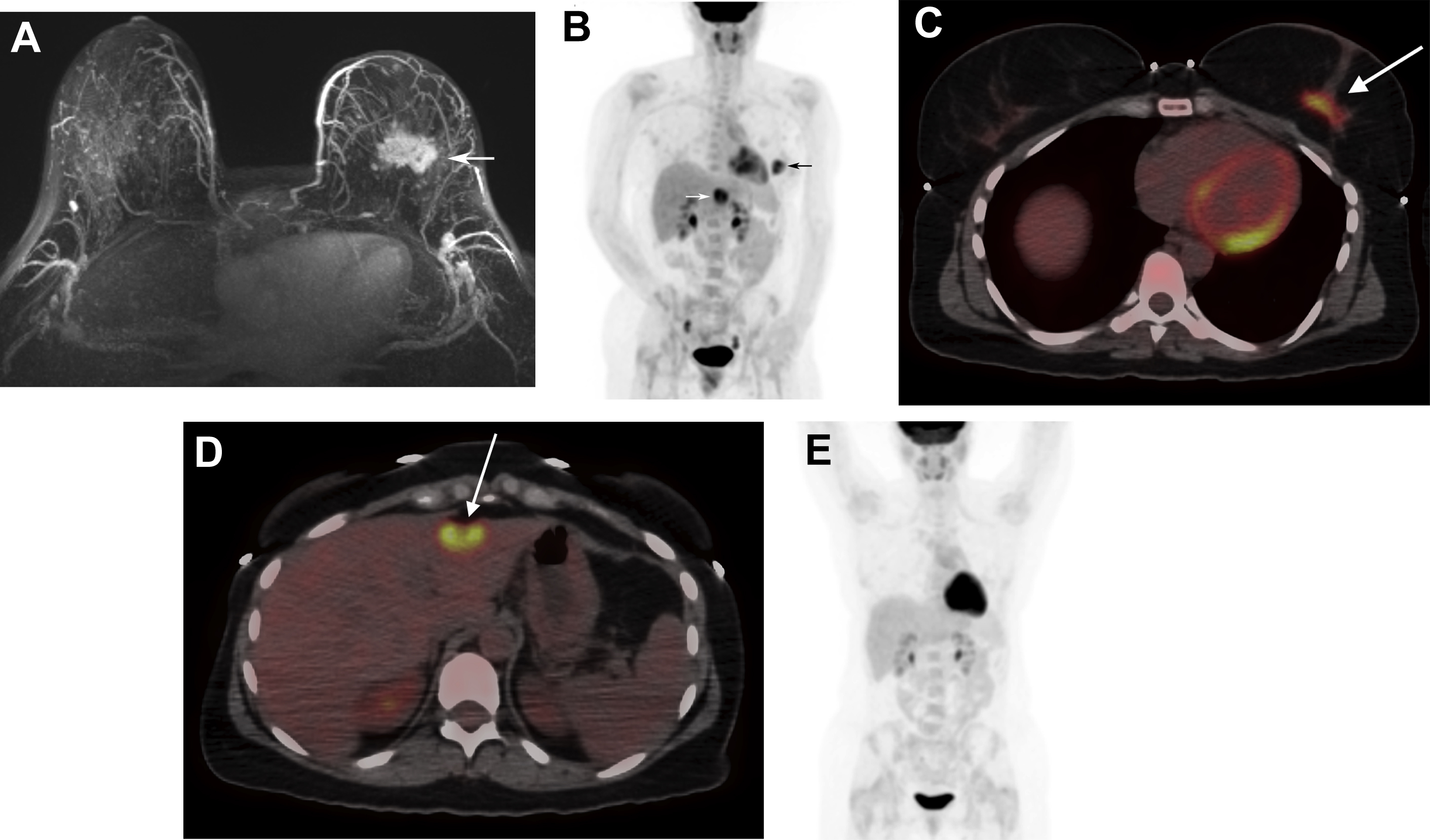
The relative utilization of conventional imaging with CT and bone scintigraphy versus FDG PET/CT for systemic staging is variable in clinical practice. NCCN guidelines consider FDG PET/CT use optional when conventional imaging is equivocal or suspicious. However, recent studies of patients with stages II–III breast cancer planning neoadjuvant chemotherapy have demonstrated that FDG PET/CT has less false-positive findings, comparable cost, and lower radiation exposure compared with conventional imaging. , Furthermore, FDG PET/CT has been shown to have higher sensitivity compared with conventional imaging for diagnosing distant metastases in patients with breast cancer. , Thus, FDG PET/CT could be considered an alternative, rather than adjunct, to CT and bone scintigraphy for systemic staging.
Consideration of additional prognostic factors beyond clinical stage may further refine the most appropriate patient population for FDG PET/CT imaging for systemic staging. These factors include histologic subtype, molecular subtype, and patient age. Hogan and colleagues demonstrated that FDG PET/CT more frequently identified unsuspected distant metastatic disease in patients with stage III invasive ductal carcinoma compared with stage III invasive lobular carcinoma. In a retrospective study of 232 patients with triple-negative breast cancer, FDG PET/CT discovered distant metastases in 15% with stage IIB disease. In a retrospective study of 144 women younger than 40 years, Riedl and colleagues demonstrated that FDG PET/CT identified unsuspected distant metastases in 17% with stage IIB disease. These data are slightly higher than the previously reported 11% yield of detecting distant metastases overall for stage IIB disease in a prospective study of 254 consecutive patients. Thus, systemic staging with FDG PET/CT may be justified starting as early as stage IIB, particularly for patients who are younger than 40 years with triple-negative invasive ductal carcinoma.
A major change in the AJCC staging system for breast cancer occurred with the 8th edition reflecting the new era of precision medicine. Biologic factors with established prognostic significance (histologic grade, ER, PR, and HER2 status, multigene panel recurrence scores) have been incorporated with the traditional anatomic stage to yield a prognostic stage for more accurate estimation of individual outcomes. Although not currently used as a biomarker in the AJCC prognostic stage, FDG PET/CT may provide additional prognostic information because breast cancers with high FDG avidity have been shown to correlate with poorer outcomes.
Suspected Disease Recurrence
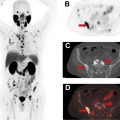
More than 3.8 million women are living in the United States with a personal history of breast cancer. Approximately, 30% to 40% of breast cancer recur as metastatic disease and the risk of recurrence continues for decades after initial treatment, particularly for patients with hormone receptor-positive breast cancer. The American Cancer Society (ACS), American Society of Clinical Oncologists (ASCO), and NCCN recommend regular clinical assessment (history and physical examination) and annual mammography for breast cancer survivors. , Breast MRI is also recommended in addition to mammography by the ACR for women with a personal history of treated breast cancer who are diagnosed before the age of 50 years or have mammographically dense breasts. Laboratory tests and systemic imaging are not recommended for asymptomatic breast cancer survivors. For patients presenting with symptoms, physical examination findings, or laboratory abnormalities suspicious for disease recurrence, systemic imaging is indicated. Imaging options include CT, MRI, bone scintigraphy, and FDG PET/CT ( Fig. 3 ).
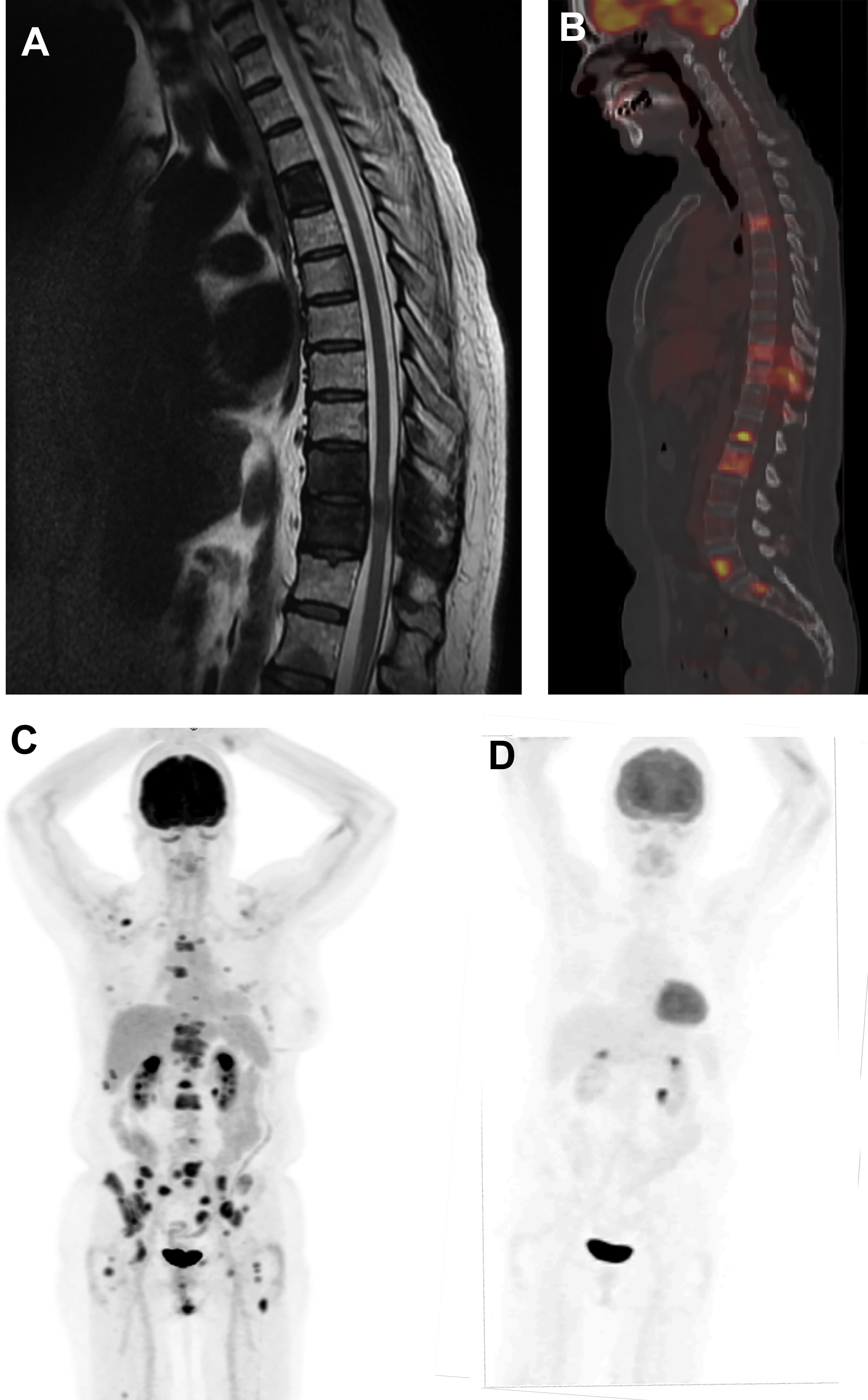
FDG PET/CT can be performed for the detection of distant metastases as well as locoregional recurrences (chest wall, axillary and internal mammary lymph nodes) in patients previously treated for breast cancer. In a meta-analysis of 26 studies of FDG PET or PET/CT with 1752 patients, summary sensitivity and specificity for detecting recurrent breast cancer was 0.90 [0.88–0.92 95% CI] and 0.81 [0.78–0.84 95% CI], respectively. FDG PET/CT has been shown to have increased sensitivity compared with CT for the diagnosis of breast cancer recurrence. FDG PET/CT may also be more accurate than contrast-enhanced CT alone or contrast-enhanced CT combined with bone scintigraphy for diagnosing breast cancer recurrences.
Treatment Response—Primary Setting
Neoadjuvant chemotherapy can be used to treat patients with breast cancer before surgery to reduce tumor size, allowing for lumpectomy instead of mastectomy, and to minimize the extent of axillary surgery. Importantly, long-term survival outcomes for patients who received neoadjuvant chemotherapy are comparable to those with adjuvant chemotherapy. As a complement to physical examination, imaging provides important information for evaluating response to neoadjuvant therapy. , Imaging before neoadjuvant chemotherapy may be helpful for predicting which patients are most likely to respond. Imaging can also be performed during therapy to confirm clinical suspicion of disease progression and to identify early nonresponders to therapy to allow for a change in treatment or to expedite surgery. Imaging performed after completion of neoadjuvant therapy can be used for planning the extent of surgery. Current standard of care is to perform surgery even if no residual imaging abnormality remains after neoadjuvant therapy because microscopic residual disease on final surgical pathology is possible.
Several meta-analyses have investigated FDG PET/CT for assessing response to neoadjuvant chemotherapy. FDG PET and PET/CT have a pooled sensitivity of 71% to 87% and specificity of 66% to 85% for determining pathologic response. For studies in which patients underwent both FDG PET/CT and breast MRI, there was comparable accuracy for predicting therapy response. Some studies found that FDG PET/CT had higher sensitivity and MRI had higher specificity for determining pathologic response. , , Although MRI may be better at assessing residual disease burden post-therapy, FDG-PET/CT may be better at assessing early response, between 1 and 3 cycles of chemotherapy. , Response to neoadjuvant chemotherapy on FDG PET/CT also has prognostic significance as it has been shown to predict disease recurrence and survival.
Treatment Response—Metastatic Setting
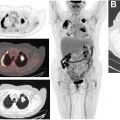
Imaging is used, together with symptom assessment, physical examination, and blood tests, to monitor for disease progression in patients undergoing treatment for metastatic breast cancer. Conventional imaging to assess treatment efficacy includes CT and/or bone scintigraphy with a suggested interval of every 2–6 months. To assess interval changes in tumor burden, standardized criteria have been established for defining complete response, partial response, stable disease, and disease progression as an objective measurement of response to therapy. Determination of disease progression is clinically significant as it triggers a change to a subsequent line of therapy.
FDG PET/CT can be used to assess response to therapy for patients with metastatic breast cancer by measuring therapy-induced changes in tumor metabolism and glycolytic activity (see Figs. 2 and 3 ). Metabolic changes typically occur earlier than changes in tumor size measurements. Reduced FDG uptake after the first cycle of chemotherapy has been shown to predict clinical response defined by conventional imaging. , Furthermore, the metabolic response after 3 cycles of chemotherapy has been shown to predict both the clinical response at the completion of 6 cycles of chemotherapy and overall survival. For bone-dominant metastatic breast cancer, changes in tumor metabolic activity may more closely reflect treatment response than CT or bone scintigraphy. Guidelines for reproducible imaging acquisition methods and standardized criteria for metabolic response assessment , are important for accurately evaluating therapy response with FDG PET/CT.
Relatively, few studies directly compare FDG PET/CT with conventional imaging for predicting survival outcomes. , In a study of 65 patients with metastatic breast cancer undergoing first- or second-line therapy, Riedl and colleagues demonstrated that metabolic response on PET/CT was a better predictor of progression-free survival and disease-specific survival than anatomic response on contrast-enhanced CT. Thus, additional prospective data are needed.
For patients with hormone receptor-positive metastatic breast cancer, FDG PET/CT has also been studied for assessing response to endocrine therapy. The metabolic response based on the difference in SUV max between FDG PET/CT performed at baseline and during endocrine therapy has been shown in small pilot studies to predict progression-free survival. , The timing of imaging is important because a transient paradoxic increase in FDG uptake (“metabolic flare”) can be observed if performed during the first 2 weeks of therapy with tamoxifen that correlates with clinical benefit.
Limitations of FDG PET/CT
FDG PET/CT is not indicated for primary breast cancer detection, for distinguishing benign from malignant breast lesions, or for local tumor staging in patients with newly diagnosed breast cancer. The spatial resolution of whole-body PET/CT scanners limits the sensitivity for detecting cancers smaller than 1 cm. Also, an inherent biologic limitation of FDG PET/CT is that breast cancers have a wide spectrum of glycolytic activity. For example, low-grade invasive carcinoma and ductal carcinoma in situ can have low FDG avidity resulting in reduced sensitivity of FDG PET/CT for detection. , Lower FDG uptake and reduced PET/CT sensitivity have also been observed for invasive lobular carcinoma compared with invasive ductal carcinoma. , Furthermore, certain benign etiologies may be FDG avid resulting in false-positive findings, such as silicone granuloma, fat necrosis, fibroadenoma, postsurgical changes, inflammation/abscess, granulomatous mastitis, gynecomastia, and lactational change.
Although FDG PET/CT has limitations for evaluating breast lesions, it is important to identify and appropriately evaluate abnormal FDG uptake in the breast. There is a 55% to 60% likelihood of malignancy (primary breast cancer, lymphoma, non-breast metastases) for incidentally detected FDG-avid breast lesions. , Thus, further evaluation is warranted, starting with correlation with recent breast imaging examinations. If none are available or if the finding is not reconciled, then diagnostic mammography and ultrasound are indicated for further evaluation.
All imaging, including FDG PET/CT, is less sensitive for detecting axillary nodal metastases compared with sentinel lymph node biopsy, the current gold standard. However, the specificity of FDG PET/CT has been reported as 0.93 [0.90–0.95 95% CI], which is superior to its sensitivity [0.64; 0.59–0.69 95% CI]. FDG PET/CT may have clinical utility for initial staging by detecting extra-axillary nodal metastases such as internal mammary and supraclavicular lymph nodes. , Internal mammary nodal metastases were equally detected by PET/CT and breast MRI in patients before neoadjuvant chemotherapy. Identification of extra-axillary nodal metastases at initial staging has prognostic significance and can impact clinical management by altering adjuvant radiation therapy planning.
PET/MRI
There is increasing interest in evaluating the clinical utility of FDG PET/MRI for patients with breast cancer for both primary disease, locoregional and distant metastatic staging. , One advantage over PET/CT is the significant reduction (50%–80%) in radiation dose with PET/MRI. , PET/MRI appears to have higher sensitivity for identifying liver and bone lesions than PET/CT. However, PET/CT may be better for detecting small lung metastases than PET/MRI , ; although the number of malignant nodules missed by PET/MRI was low (0.8%). The diagnostic performance of PET/MRI for pulmonary metastases may improve as further technical development of motion-robust MRI sequences for lung imaging continue to evolve.
FES PET/CT
In 2020, the United States Food and Drug Administration approved FES for clinical use with PET imaging to detect ER-positive lesions in patients with recurrent or metastatic breast cancer as an adjunct to biopsy. This is a major achievement because FES has been studied in more than one thousand patients participating in research since 1988. A historical perspective on the discovery and development of FES was recently published.
FES is a radiolabeled estrogen that can be used together with PET imaging to determine the location and ligand-binding function of ER throughout the body. Several studies have demonstrated concordance between FES PET imaging results and ER status in primary and metastatic breast cancer using tissue reference standards. Proposed clinical applications of FES PET/CT include the detection of ER+ metastases, evaluation of suspected recurrence in patients with a history of ER+ primary breast cancer, problem-solving when conventional work-up is inconclusive, and therapy selection.
FES PET/CT can be performed for post- and pre-menopausal women, as well as men, with breast cancer. The recommended injected dose of FES is 6 mCi (222 MBq; 111–222 MBq; 3–6 mCi). Uptake time before scanning is typically 60 minutes (50–70 minutes), similar to FDG. Unlike FDG PET imaging preparation, fasting before injection and the limitation of physical activity is not required for FES PET/CT. FES PET/CT can be performed while patients are taking aromatase inhibitors, but not with ER antagonists such as tamoxifen or fulvestrant due to competitive blocking of ER for FES binding. Owing to the normal hepatobiliary clearance of FES, there is limited evaluation of liver lesions and abdominal lymph nodes located near bowel with high physiologic clearance activity.
Ongoing research
There are many exciting ongoing investigations in the area of molecular imaging for breast cancer. For example, radiopharmaceuticals for in vivo measurement of other clinically established tumor biomarkers beyond ER are being developed and evaluated in clinical trials. These investigational imaging agents include 18 F-fluorofuranylnorprogesterone ( 18 F-FFNP) for progesterone receptor, 89 Zr-trastuzumab or 89 Zr-pertuzumab for HER2, and 18 F-fluorothymidine ( 18 F-FLT) and 18 F-ISO-1 for proliferation. Furthermore, new approaches involving radiomics, texture analysis, and deep learning may offer additional prognostic and predictive information. Lastly, dedicated breast PET imaging devices and simultaneous breast PET/MRI protocols have been developed that may expand the utility of multimodality molecular imaging for primary breast tumor evaluation. ,
Clinics care points
- •
FDG PET/CT can be used for systemic staging of breast cancer, evaluation of suspected disease recurrence, and assessment of treatment response.
- •
Potential causes for a false negative FDG PET/CT examination include ductal carcinoma in situ, invasive lobular carcinoma, and subcentimeter cancers.
- •
Abnormal FDG uptake in the breast incidentally detected on PET/CT has a high likelihood of malignancy and warrants further evaluation.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree