Key points
- •
SSTR-PET has significantly improved staging of NETs compared to historical radiotracers.
- •
Knowledge of NET pathology is essential for selection of appropriate PET radiotracer.
- •
PET imaging with both SSTR-PET tracers and FDG may play a significant role in guiding treatment decisions for patients with moderately or poorly differentiated NETs.
Introduction
Neuroendocrine tumors (NETs) consist of a wide array of lesions arising from multiple organs throughout the body with an estimated 12,000 people diagnosed in the United States each year and approximately 170,000 people alive with a known diagnosis of NET. These tumors arise from cells in both the endocrine and nervous systems and can demonstrate varying levels of hormone secretion, which may elicit symptoms that prompt their discovery. Most commonly, NETs arise from the bowel, pancreas, and lung but can be found in multiple additional organs. Typically, these are first discovered through a combination of anatomic imaging with either computed tomography (CT) or MR imaging and endoscopy with or without endoscopic ultrasound (EUS). Once diagnosed, accurate staging and tumor grading is needed to determine the optimal treatment algorithm for these patients, as they can range from slow-growing and indolent to highly aggressive with a propensity to metastasize.
Traditionally, molecular imaging has played a large role in the noninvasive diagnosis of NETs through the use of [ 111 In]pentetreotide scintigraphy with planar gamma camera imaging and single positron emission computed tomography (SPECT), as this radiotracer targets somatostatin receptors (SSTRs) that are overexpressed on NETs in varying degrees. In 2016, the United States Food and Drug Administration (FDA) approved [ 68 Ga]DOTATATE as a PET radiotracer for PET imaging of NETs, with improved performance of this agent over planar and SPECT somatostatin receptor scintigraphy. , In addition, in 2018, the United States FDA approved a targeted systemic therapy ([ 177 Lu]DOTATATE) to somatostatin-receptor-expressing gastroenteropancreatic NETs, using a theranostic approach where patients are first imaged with [ 68 Ga]DOTATATE to noninvasively determine SSTR expression and subsequently treat with a therapeutic radioisotope targeting the exact same receptor used in PET imaging. These advances have led to substantial increases in PET imaging utilization for NETs, and thus, knowledge of strengths and weaknesses of PET imaging of NETs is essential to both the general radiologist and subspecialty radiologist. The focus of this article is current and future applications of PET in several of the most common NETs, current PET radiotracers available for NET imaging, and pathologic considerations in molecular imaging of NETs.
Tumor grading and impact of tumor pathology on PET imaging
As stated previously, NETs are a heterogeneous group of tumors with varying degrees of aggressiveness and differentiation. In 2017, the World Health Organization (WHO) revised the pathologic classification of pancreatic NETs (PNETs) which was further adapted to NETs of other origin in 2019. NETs, or neuroendocrine neoplasms (NENs), are classified on the basis of differentiation, tumor grade, mitotic rate, and Ki-67 index. NENs are then categorized into NETs grades 1 to 3, neuroendocrine carcinoma (small-cell and large-cell variants), and mixed neuroendocrine–non-neuroendocrine neoplasms (MiNENs) with pathologic criteria outlined in Table 1 . Most MiNENs are mixed adenoneuroendorine carcinomas, with pathologic features of both tumors, and can occur at any primary site that both neuroendocrine and adenocarcinoma can arise. Many NETs that are initially diagnosed on EUS may be diagnosed by fine-needle aspiration, which may limit pathologic classification or cause inaccurate tumor grading. Thus, EUS-guided fine-needle core biopsies are preferable, providing important additional information to the cytologic evaluation and improving diagnostic sensitivity. Accurate pathologic characterization of NETs is an essential topic for guiding molecular imaging, as tumor grade can have a significant impact on choice of radiotracers. Well-differentiated NETs are more likely to maintain expression of SSTRs and are best imaged with PET SSTR ligands while poorly differentiated NECs are more likely to not express SSTRs but instead are best imaged with 2-deoxy-2-[ 18 F]fluoro- d -glucose (FDG) PET because of their high mitotic count and increased glucose utilization. Thus, a collaborative multidisciplinary approach between several departments should be taken to optimize diagnosis, imaging, and management of patients with NETs.
| Terminology | Differentiation | Grade | Mitotic rate (mitoses/2 mm 2 ) | Ki-67 Index |
|---|---|---|---|---|
| Neuroendocrine tumor | ||||
| Grade 1 (G1) | Well | Low | <2 | <3% |
| Grade 2 (G2) | Well | Intermediate | 2–20 | 3%–20% |
| Grade 3 (G3) | Well | High | >20 | >20% |
| Neuroendocrine carcinoma | ||||
| Small cell | Poor | High | >20 | >20% |
| Large cell | Poor | High | >20 | >20% |
| MiNEN | Variable (usually poor) | Variable | Variable | Variable |
PET radiotracers, biodistribution, and imaging protocols
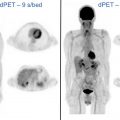
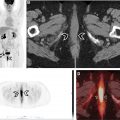
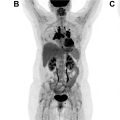
Historically, molecular imaging has long played a role in the imaging of NETs. The earliest imaging agent, [ 111 In]pentetreotide, targeted SSTRs at the surface of the cell, mainly subtypes SSTR2 and SSTR5. These SSTRs (predominantly SSTR2) are overexpressed on the surface of NET cells and are preferentially imagined through this mechanism. [ 111 In]Pentetreotide imaging involved a combination of planar, SPECT, and SPECT/CT imaging to accurately diagnose and localize NETs. Building on this same principle, several PET radiotracers have been developed for improving imaging of NETs ( Table 2 ) because of their improved spatial resolution and signal-to-noise ratio when compared with SPECT imaging agents. Notably, all DOTA-peptide radiotracers demonstrate a higher affinity for SSTR2 than [ 111 In]pentetreotide, which improved detection and accurate characterization of lesions. Knowledge of normal radiotracer biodistribution is important to avoid interpretation errors. For [ 68 Ga]DOTATATE and similar SSTR ligands, the spleen demonstrates the highest level of radiotracer activity, followed by the adrenal glands, pituitary, kidneys, and liver ( Fig. 1 ). Less activity is noted in the salivary and thyroid glands with variable amounts of activity noted in the bowel.
| Name | SSTR affinity | Half-life | Production | Approval status |
|---|---|---|---|---|
| [ 68 Ga]DOTATATE | SSTR2 | 68 min | Ga/Ge generator | Yes (2016) |
| [ 68 Ga]DOTATOC | SSTR2, SSTR5 | 68 min | Ga/Ge generator | Yes (2019) |
| [ 68 Ga]DOTANOC | SSTR2, SSTR5>SSTR3 | 68 min | Ga/Ge generator | No |
| [ 64 Cu]DOTATATE | SSTR2 | 12.7 h | Cyclotron | Yes (2020) |

Currently, SSTR-PET/CT and PET/MR imaging are used for several clinical scenarios including localization of NET of unknown primary, staging of patients with known NET, follow-up for patients with NET to evaluate response to treatment and/or progression, and for noninvasive determination of SSTR expression and potential treatment with peptide receptor radionuclide therapy (PRRT). Unlike [ 18 F]FDG-PET/CT, there is no need for patients to fast before SSTR-PET/CT, as blood sugar levels do not alter tracer distribution. Many patients with known diagnoses of NETs are frequently on long-acting somatostatin analogs to treat their symptoms, and there is a theoretic potential for these nonradiolabelled analogs to interfere with and decrease sensitivity of SSTR-PET. Thus, some advocate for timing the SSTR-PET scan just before the next scheduled injection. However, a recent prospective study found that lanreotide had a minimal effect on DOTATATE uptake in tumors and should not influence timing of the PET scan. Adult patients are typically administered an intravenous dose of DOTATATE between 2.7 and 5.4 mCi (100–200 MBq), and the typical uptake period is approximately 60 minutes. Patients are encouraged to void before scanning to minimize radiation dose to the urinary bladder and decrease activity in the pelvis. Once on the PET scanner, patients are scanned typically from skull base to mid-thigh using 3- to 5-minute PET bed positions.
Gastroenteropancreatic neuroendocrine tumors
Gastroenteropancreatic neuroendocrine tumors (GEP-NETs) are the most common type of NET and account for approximately 70% of NETs, with an estimated incidence of 3.56 per 100,000 and accounting for 2% of all primary gastrointestinal and pancreatic neoplasms. , Most GEP-NETs occur sporadically, but these tumors are associated with multiple genetic syndromes, including multiple endocrine neoplasia type 1 (MEN-1), neurofibromatosis type 1, Von Hippel-Lindau disease, and tuberous sclerosis. The gastrointestinal tract is the most common site of NET origin, accounting for 67% of cases. Once thought to be a rare tumor, PNETs are being diagnosed more frequently in the past several decades, likely due to an increase in medical imaging, as many of these tumors (70%–90%) are hormonally nonfunctional and are discovered incidentally. Owing to the lack of symptoms, a significant number of GEP-NETs are diagnosed late and are at an advanced stage at presentation. Functional NETs tend to present earlier, and symptoms can differ depending on tumor type, with insulinomas and gastrinomas being most common. Carcinoid syndrome is classically associated with small bowel NETs but occurs in the minority of patients with nonmetastatic small bowel NETs (6%–30%) and the presence significantly increasing in the setting of hepatic metastases (95% of cases). While the initial diagnosis of GEP-NET often is made on conventional imaging with CT and MR imaging, molecular imaging has long played a role in the accurate diagnosis and staging of these patients with [ 68 Ga]DOTATATE PET/CT offering significant improvement over [ 111 In]pentetreotide scintigraphy.
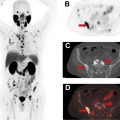
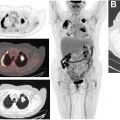
Several studies and meta-analyses have directly compared the performance of [ 68 Ga]DOTATATE PET/CT and [ 111 In]pentetreotide scintigraphy. An early study evaluating the use of [ 68 Ga]DOTATATE PET/CT in patients with known NETs (predominantly GEP-NETs) found that [ 68 Ga]DOTATATE PET/CT identified significantly more lesions than [ 111 In]pentetreotide scintigraphy and changed management of 36/51 patients (70.6%). In a study by Deppen and colleagues, [ 68 Ga]DOTATATE PET/CT performed equally or superior to [ 111 In]pentetreotide scintigraphy in all patients (n = 78) with a sensitivity and specificity of 96% and 93%, respectively, for [ 68 Ga]DOTATATE PET/CT compared with 72% and 93%, respectively, for [ 111 In]pentetreotide scintigraphy. In addition, this study found that [ 68 Ga]DOTATATE PET/CT correctly identified 3 patients for potential PRRT that were inaccurately classified by [ 111 In]pentetreotide. This same study also examined safety of the [ 68 Ga]DOTATATE radiotracer and found no instances of a trial-related event requiring treatment. A separate prospective study evaluating the use of [ 68 Ga]DOTATATE PET/CT, [ 111 In]pentetreotide SPECT/CT, multiphasic CT, and/or MR imaging for detection of unknown primary and metastatic GEP-NETs found that [ 68 Ga]DOTATATE PET/CT detected 95.1% of lesions, compared with anatomic imaging detecting 45.3%, and [ 111 In]pentetreotide SPECT/CT detecting 30.9% of lesions. A 2016 meta-analysis of the literature estimated a sensitivity of 90.9% and specificity of 90.6% for [ 68 Ga]DOTATATE PET/CT for the diagnosis and staging of NETs ( Fig. 2 ). As a result of this superior performance, both the North American Neuroendocrine Tumor Society (NANETS) and National Comprehensive Cancer Network (NCCN) include [ 68 Ga]DOTATATE PET/CT as a recommendation for staging patients with newly diagnosed GEP-NET ( Fig. 3 ). ,