MR imaging and PET using 2-Deoxy-2-[ 18 F]fluoroglucose (FDG) are both useful in the evaluation of gynecologic malignancies. MR imaging is superior for local staging of disease whereas fludeoxyglucose FDG PET is superior for detecting distant metastases. Integrated PET/MR imaging scanners have great promise for gynecologic malignancies by combining the advantages of each modality into a single scan. This article reviews the technology behind PET/MR imaging acquisitions and technical challenges relevant to imaging the pelvis. A dedicated PET/MR imaging protocol; the roles of PET and MR imaging in cervical, endometrial, and ovarian cancers; and future directions for PET/MR imaging are discussed.
Key points
- •
MR imaging and PET individually play important and complementary roles in assessing gynecologic malignancy.
- •
MR imaging is the modality of choice for assessing local extension of tumors, whereas PET adds value for detection of distant metastases.
- •
Simultaneous PET/MR imaging systems combine these advantages while reducing radiation dose compared with PET/CT.
- •
Novel MR imaging and PET tracers promise to extend the applications of PET/MR imaging.
Introduction
MR imaging and PET play vital roles in evaluating patients with gynecologic malignancies. MR images of the pelvis provide excellent spatial resolution and soft tissue contrast to precisely define the local extent of pelvic tumors. Diffusion-weighted imaging (DWI) and dynamic contrast enhancement (DCE) provide additional information about tissue properties. PET, which is usually performed using the glucose analog 2-Deoxy-2-[ 18 F]fluoroglucose (FDG), is valuable for detecting distant metastases. Gynecologic malignancies are, therefore, natural applications for simultaneous PET/MR imaging acquisitions, which combine the advantages of both modalities into a single scan.
The goal of this article is to review the ways that PET/MR imaging acquisitions can be applied to the evaluation of gynecologic malignancies. The technical challenges of performing a PET/MR imaging scan are reviewed focusing on how these challenges have an impact on the evaluation of gynecologic malignancies. The role of PET and MR imaging alone in the evaluation of cervical cancer, endometrial cancer, and ovarian cancer is discussed. Current efforts to combine the information between two scans, including exciting initial results using dedicated PET/MR imaging systems, are discussed. Finally, future directions for this rapidly developing technology, including its use with novel emerging MR imaging and PET contrast agents, are discussed.
Introduction
MR imaging and PET play vital roles in evaluating patients with gynecologic malignancies. MR images of the pelvis provide excellent spatial resolution and soft tissue contrast to precisely define the local extent of pelvic tumors. Diffusion-weighted imaging (DWI) and dynamic contrast enhancement (DCE) provide additional information about tissue properties. PET, which is usually performed using the glucose analog 2-Deoxy-2-[ 18 F]fluoroglucose (FDG), is valuable for detecting distant metastases. Gynecologic malignancies are, therefore, natural applications for simultaneous PET/MR imaging acquisitions, which combine the advantages of both modalities into a single scan.
The goal of this article is to review the ways that PET/MR imaging acquisitions can be applied to the evaluation of gynecologic malignancies. The technical challenges of performing a PET/MR imaging scan are reviewed focusing on how these challenges have an impact on the evaluation of gynecologic malignancies. The role of PET and MR imaging alone in the evaluation of cervical cancer, endometrial cancer, and ovarian cancer is discussed. Current efforts to combine the information between two scans, including exciting initial results using dedicated PET/MR imaging systems, are discussed. Finally, future directions for this rapidly developing technology, including its use with novel emerging MR imaging and PET contrast agents, are discussed.
Technical factors
Technical Challenges of the PET/MR Imaging Acquisition
Detection
Combining PET and MR imaging into a single acquisition is technically challenging. PET agents, such as FDG, emit positrons, which interact with nearby electrons and emit pairs of high-energy photons. Traditionally, the photons emitted during a PET scan have been detected using photomultiplier tubes, which are sensitive to large magnetic fields and, therefore, are inappropriate for an MR imaging environment. This problem has been solved by the development of solid state PET detectors, including avalanche photodiodes, which are not as sensitive to the presence of magnetic fields.
Attenuation correction
High-energy photons that are produced within the body during PET scans are attenuated by overlying soft tissue, causing structures deep within the body to have lower signal intensities than structures closer to the surface. PET images require attenuation correction to adjust the signal intensity to account for differing amounts of attenuation. In integrated PET/CT scans, the CT acquisition serves as a direct reference for the amount of x-ray attenuation. Because MR imaging acquisitions are not x-ray based, they do not provide a direct attenuation reference. Therefore, alternative approaches must be used.
One of the most popular methods for MR imaging–based attenuation correction relies on a Dixon-based technique, where two images are acquired, one with fat and water signals in phase and one with fat and water signal out of phase. Linear combinations of these two images generate maps of the fat and water content of the body. Segmenting the body into fat, soft tissue, and lungs allows an attenuation map to be generated.
Accounting for the attenuation of cortical bone remains a challenge for MR imaging–based attenuation correction methods. Cortical bone produces significant x-ray attenuation but has very low signal on MR imaging scans. This potentially leads to error in quantification of standardized uptake values (SUVs), potentially leading to a 10% to 18% underestimation of SUVs. This is expected to be particularly problematic within the pelvis, which is surrounded by bone.
Accounting for cortical bone during attenuation correction is an evolving area of research. One approach uses predetermined atlases to correct for how much cortical bone is expected in each patient. Another approach relies on acquisition of a specialized MR imaging pulse sequence with either ultrashort or zero echo time. In these sequences, cortical bone signal can be measured and, therefore, accounted for in attenuation correction maps.
Lung nodules
Lung imaging using MR imaging is challenging. Because air within the lung creates numerous areas of varying magnetic susceptibility, lung tissue has a very short T 2 * and, therefore, low signal on most MR imaging pulse sequences. In addition, the spatial resolution of MR imaging acquisitions tends to be much lower than CT acquisitions. Both of these facts cause MR imaging to be less sensitive to small lung nodules compared with CT. Ultrashort-echo and zero-echo time pulse potentially could be used to detect smaller nodules, but this reduced sensitivity to small lung nodules remains a limitation of PET/MR imaging compared with PET/CT. The clinical importance of reduced sensitivity to small lung nodules has yet to be determined and likely varies with primary cancer type.
Respiratory and bowel motion
Respiratory and bowel motion occur continuously during the PET/MR imaging acquisition and are particularly significant when acquiring the abdomen and upper pelvis. Because the MR imaging acquisition is significantly longer than the CT acquisition, this can potentially have an impact on the anatomic localization of small peritoneal implants. Because MR imaging data are being acquired throughout the acquisition, the MR imaging data potentially serve as a reference for correcting for motion in the PET acquisition, leading to improved image quality.
Bladder filling
The urinary bladder filling over the course of an PET/MR imaging acquisition may cause changes in position of the uterus between the beginning and end of the acquisition. Therefore, it is especially important that the PET and MR imaging acquisitions occur simultaneously. Otherwise, significant errors could occur in the localization of uterine tumors in particular.
MR Imaging Sequences for Gynecologic Malignancies
MR imaging of gynecologic malignancies is based on high-resolution imaging of the local extent of tumor, together with characterization of the chemical composition of tumor using fat-sensitive sequences. Further characterization of the tissue properties of tumors can be obtained using DWI and DCE (described later).
High-resolution T 2 -weighted imaging
The most important pulse sequences for imaging the female pelvis are high-resolution T 2 -weighted fast spin-echo (FSE) pulse sequences performed without fat saturation. The use of non–fat-saturated sequences allows for precise definition of tissue planes and serosal margins. T 2 -weighted imaging also permits identification of fluid collections and ascites. For patients with cervical and endometrial malignancies, it can be useful to choose image planes that are perpendicular to the axis of the tumor to better assess parametrial or endometrial invasion. There is currently interest in replacing traditional 2-D FSE pulse sequences with novel 3-D T 2 -weighted FSE sequences (CUBE [GE Healthcare, Waukesha, WI] and T 2 SPACE [Siemens Medical Systems, Erlangen, Germany]). Because 3-D FSE sequences obtain volumetric data they can, in principle, be reformatted into multiple image planes. Further research is required to determine if these reconstructed sequences have the same image contrast properties and diagnostic utility as their primarily acquired 2-D T 2 counterparts.
T 1 -weighted gradient-echo sequences
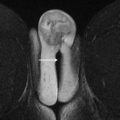
Multiecho T 1 gradient-echo sequences acquired without fat saturation are obtained with a field of view that encompasses the entire pelvis. Microscopic fat within lesions can be detected by measuring signal loss that occurs when fat and water are acquired with their signals out of phase. Macroscopic fat can be detected by looking for “India ink” artifact that occurs at lesion boundaries or by comparing T 1 -weighted images acquired with and without fat saturation. Because blood products have intrinsically high signals on T 1 -weighted sequences, these pulse sequences can aid in the diagnosis of endometriomas ( Fig. 1 A–C ). The presence of macroscopic fat permits the diagnosis of definitively benign lesions, such as dermoid cysts ( Fig. 1 D–F).

Contrast-enhanced spoiled gradient echo
T 1 -weighted spoiled gradient-echo (SPGR) images with fat saturation can be obtained in a high-resolution volumetric acquisition and are typically acquired before and after administration of gadolinium contrast. Among many other uses, gadolinium enhancement can enhance the conspicuity of endometrial masses relative to the myometrium and identify solid enhancing nodules within adnexal masses. Contrast-enhanced images are generally acquired in multiple contrast phases, which can vary in terms of tumor conspicuity.
Diffusion-weighted imaging
DWI has gained increasing attention in characterizing gynecologic malignancies. In DWI, image contrast is related to how freely water molecules can diffuse within tissues. Molecules that are in a more restrictive environment have higher signal on DWI and molecules that can move more freely have lower signal on DWI. The diffusion properties of tissues are quantified by computing the apparent diffusion coefficient (ADC), which is the rate that signal decays across multiple DWIs. Because the ADC is, in principle, an intrinsic property of the material being studied, there is great interest in using ADC as a quantitative biomarker of disease. Many small studies have suggested that low ADC may be related to tumor aggressiveness and used to predict malignancy. There is still considerable overlap, however, between benign and malignant tumors. DWI has also been shown to make peritoneal metastases more conspicuous. Because DWI and PET probe different properties of tissues, namely metabolic activity and cell density, it is hoped that through PET/MR imaging examinations they may provide complementary information about tumors.
PET/MR Imaging Protocol
At the authors’ institution, simultaneous PET/MR imaging examinations are performed using a system based on a 3T wide-bore MR imager integrated with a dedicated time-of-flight PET inset (Signa, GE Healthcare, Waukesha, WI). PET and MR imaging data are acquired simultaneously. For gynecologic malignancy evaluation, the authors use a combined whole-body acquisition and a dedicated pelvis bed position ( Fig. 2 ). The dedicated pelvis acquisition uses the pulse sequences described in the previous section, with high-resolution T 2 -weighted FSE sequences in 3 planes, obliqued with respect to the cervix or uterus, depending on the location of the tumor (see Fig. 2 B). High-resolution T 2 -weighted images are obtained with 4-mm slices and voxels that are 0.6 mm to 0.9 mm on a side. A small field-of-view diffusion-weighted acquisition is acquired, covering the primary tumor only (slice thickness 5 mm and voxel size 1.5 mm × 1.5 mm; see Fig. 2 C). Subsequently, a time-resolved 3-D gradient-echo T 1 -weighted image SPGR acquisition is acquired for evaluation of DCE (see Fig. 2 D). For ovarian masses, a 2-D dual-echo gradient echo is added to evaluate for fat.

For the whole-body PET and MR imaging portion of the examination (see Fig. 2 E–F), each bed position is imaged for 3 minutes, and simultaneously a postgadolinium T 1 -weighted SPGR image is acquired in the axial plane as well as single-shot FSE (SSFSE) acquisitions in the axial and coronal plane. T 1 -weighted SPGR images are acquired using a dual-echo Dixon-based approach that can then be used to produce fat and soft tissue maps that are used for attenuation correction.
The dedicated pelvis acquisition takes approximately 20 minutes in total to acquire, and the whole-body acquisition requires an additional 20 minutes. Patient set up (coil placement, localizer acquisition, and plane placement) takes between 10 minutes and 15 minutes, resulting in a total acquisition time of approximately 60 minutes.
One subtle but important detail of the PET/MR imaging acquisition is that the isocenter of the MR image acquisition must be the same as the center of the bed position of the PET acquisition. For optimal image quality, however, the isocenter is ideally placed at the location of the tumor. For this reason, the authors also acquire a sagittal T 2 -weighted SSFSE image of the pelvis prior to beginning the PET acquisition to identify the tumor location and determine the best location selection of the pelvis PET bed position (see Fig. 2 A). During the remainder of the study, PET and MR data are acquired simultaneously.
Image interpretation
Physiologic Uptake
When interpreting PET images of the female pelvis, it is important to understand the physiologic variations in FDG uptake that may occasionally mimic disease. In premenopausal patients, endometrial uptake varies cyclically with the menstrual cycle, with increased SUVs seen in menstruating and ovulating patients and lower SUVs seen in proliferative and secretory phases. The presence of an intrauterine device has also been noted to cause increased FDG uptake in the endometrium.
Abnormal ovarian uptake can be seen during ovulation, which can be potentially avoided by scheduling PET examinations just after menstruation. In premenopausal women, corpus luteum cysts avidly take up FDG and can mimic a metastatic lymph nodes, particularly on PET/CT examinations that are performed without CT contrast. Ovarian hyperstimulation in the setting of oocyte retrieval has been noted to cause increased FDG uptake and should not be confused with malignancy. In contrast, increased ovarian uptake in postmenopausal women should be taken as a sign of malignancy.
In addition to the ovaries and endometrium, fallopian tubes demonstrate increased FDG uptake in the midportion of the menstrual cycle. Inflammatory processes in the fallopian tubes, such as salpingitis, can also cause increased FDG uptake ( Fig. 3 ). Physiologic uptake in the fallopian tubes can be particularly problematic in light of emerging evidence that the fallopian tubes may represent the site of origin for serous ovarian cancers.

Combined PET and MR Imaging in Gynecologic Malignancies
These sections review the current literature regarding the use of combined PET and MR imaging in cervical, endometrial, and ovarian cancer. The discussion focuses mainly on studies that directly compare MR imaging and PET/CT and numerous emerging reports describing simultaneous PET/MR imaging acquisition. For each malignancy, discussion is separated into the use of these imaging modalities in the initial diagnosis, detection of recurrence, and treatment response. Because experience with this emerging modality is in the early stages, it is difficult to draw general conclusions, especially because MR imaging and PET/CT are not monolithic entities, and the performance of each depends on the protocol chosen. In particular, the CT portion of the PET/CT examination can be performed with or without contrast, and that choice affects the comparison with MR imaging for anatomic localization. Often, when PET/CT is compared with MR imaging for characterization of lymph node metastasis, MR imaging characterization relies on morphologic criteria (size >1 cm) and not other functional parameters (DWI and DCE) often used in clinical practice.
Cervical Cancer
Worldwide, cervical cancer is the fourth most common cancer in women, with a large majority of cases and deaths occurring in developing countries (2014 World Health Organization Cancer fact sheet). Most cervical cancers are associated with the human papilloma virus and approximately 12,990 cases of cervical cancer are expected in the United States in 2016. Tumors that are confined to the cervix and less than 4 cm (International Federation of Gynecology and Obstetrics [FIGO] stage IB1 or less) are generally treated with surgery, whereas those with locally advanced disease (parametrial or organ invasion) are generally treated with primary chemoradiotherapy. Tumors confined to the cervix but greater than 4 cm (stage IB2) may be treated with either surgery or chemoradiotherapy, depending on clinical characteristics, such as depth of cervical stromal invasion and concern for nodal involvement. National Comprehensive Cancer Network (NCCN) guidelines in 2016 advocate whole-body PET/CT for locally advanced disease (FIGO stage II or above) and for patients where cervical cancer is found incidentally after hysterectomy. MR imaging is considered optional for assessment of local extent of disease.
Initial staging
MR imaging and PET are complementary in the evaluation of cervical cancer, with MR imaging more sensitive for local disease and PET/CT better for evaluation of metastatic disease. MR imaging has been shown in a large multicenter study to be more accurate than CT in detecting cervical tumors and has a negative predictive value of approximately 100% in excluding bladder and rectal involvement. In a large meta-analysis, PET was found to have a higher pooled sensitivity for metastatic lymph nodes than either MR imaging or CT. In this analysis, the pooled sensitivity for PET was still low (0.58), indicating there is extensive disease that potentially remains occult on any imaging modality.
Where the combination of PET and MR imaging has been specifically evaluated, it has been shown valuable in staging of cervical cancer. In a study of 27 patients with cervical cancer using an integrated PET/MR imaging system, PET/MR imaging had high sensitivity and specificity for vaginal invasion, parametrial extension, and lymph node involvement. Both ADC and maximum SUV were correlated with tumor size and pathologic grade. A similar correlation of ADC and maximum SUV was found in a study of separately acquired PET and MR imaging scans of patients with cervical cancer. In locally advanced cervical cancer (FIGO stages IB–IVA), fused PET and MR imaging allows the detection of more lymph node groups than PET alone.
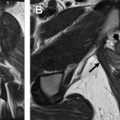
Fig. 4 shows examples of a primary cervical cancer lesion too small to reliably identify on T 2 -weighted imaging but visible using the PET overlay. Fig. 5 shows FDG uptake in a small anterior cervical mass without parametrial extension.


Prognosis
Both FDG PET and MR imaging have been shown to have value in predicting the prognosis of patients diagnosed with cervical cancer. The volume of tumor burden determined by FDG PET is correlated with overall survival after radiation therapy. A nomogram has been created based on PET characteristics to predict overall survival and progression-free survival. In a meta-analysis, high maximum SUV in both the primary tumor and metastasis has been shown to predict unfavorable prognosis. Combining PET and MR imaging into a single prognostic model provides further prognostic value compared with each study alone; aortic lymph node involvement (seen by PET/CT) and parametrial extension (seen by MR imaging) were both found powerful independent predictors of progression free survival.
Treatment response
Evaluating treatment response is particularly important for cervical cancer because advanced disease is typically treated nonoperatively. Residual FDG uptake after radiation therapy has been shown to predict a 5-year survival of 32%, compared with 80% if no uptake was seen. In a prospective study, FDG uptake on PET performed 3 months after therapy was found to predict overall survival. MR imaging also has an important role in treatment planning for brachytherapy and monitoring response. In examining the prediction of treatment response, both the change in maximum SUV and change in ADC have been found predictive of disease progression after radiation therapy.
Surveillance
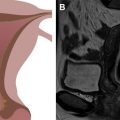
PET imaging is sensitive and specific for detection of recurrent cervical cancer, with correlative imaging, such as CT or MR imaging, helping to elucidate false-positive or false-negative PET findings. Fig. 6 shows examples of 2 different patients with recurrent cervical tumors in the vagina (see Fig. 6 A–C) and along the pelvic sidewall (see Fig. 6 D–F). In a meta-analyses of prior studies, other investigators have questioned the added value of PET/CT compared with MR imaging or CT alone, including its cost-effectiveness. The decreased radiation dose of PET/MR imaging compared with PET/CT is a major strength for surveillance imaging, particularly in young women, where multiple serial scans may be required.


Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree