Study
Number of patients
Entry criteria
photosensitizer
Summary of results
Reported adverse effects
Recurrent disease treatment
Nathan (2002)
14
Phase I/II study—patients with rising PSA and biopsy-proven local recurrence after radiotherapy
Meso-tetra hydroxyl phenyl chlorin; mTHPC (temoporfin/ ‘Foscan’)—0.15 mg/kg—high light doses used in 13/14 patients
9/13 patients who received the higher light dose showed a PSA reduction 3–6 months after treatment, and biopsies were negative in 4. All men eventually showed PSA rise—10 required androgen therapy
Acute urinary retention-3; stress urinary incontinence-2; recto-urethral fistula (after rectal biopsy)-1; photosensitivity reactions-5; erectile dysfunction-4/7 with previously acceptable function; irritative urinary symptoms-all
17
Phase I study—locally recurrent prostate cancer
Motexafin lutetium
Greatest delay to biochemical progression in the cohort treated with 2 mg/kg, fluence 150 J/cm2 and drug-light interval 3 h
Urinary urgency-1; irritative urinary symptoms
24 (2 fibres)
28 (multiple fibres—up to 6)
Phase I/II clinical trials—radio-recurrent prostate cancer Gleason >6, life expectancy >5 years, no evidence of metastases, PSA <20 ng/ml and prostate volume <50 cm3
WST-09 (Padoporfin/TOOKAD)—drug dose-escalation protocol (0.1–2 mg/kg) with light dose 100 J/cm and light dose escalation with 2 mg/kg drug dose at 230–360 J/cm
Feasibility of whole-gland treatment demonstrated—for complete response at 6-month biopsy they suggest light doses of ≥23 J/cm2 in ≥90 % prostate
Recto-urethral fistulae-2; intra-operative hypotension; irritative urinary symptoms
Primary disease treatment
Windahl (1990)
2
First clinical use of PDT in prostate cancer—patients underwent TURP 6 weeks prior to treatment
Each treated with different agent—haematoporphyrin derivative and porfimer sodium
Both showed decreasing PSA and had negative biopsy at 3 months after treatment
None reported
Moore (2006)
6 (total 10 treatments)
Phase I study—localised prostate cancer
Meso-tetra hydroxyl phenyl chlorin; mTHPC
After 8/10 sessions PSA reduced by up to 67 %, early MRI showed patchy necrosis and oedema
Sepsis-1; recatheterisations-2; temporary erectile dysfunction-1; irritative urinary symptoms-all
Zaak (2003)
6
1st treatment during radical prostatectomy, then transurethrally after TURP and then transperineally with US guidance
5-aminolevulinic acid (5-ALA)—20 mg/kg
Average PSA reduction at 6/52 after treatment: 55 % (transurethral) and 30 % (transperineal)
None reported
PCM201—40
PCM203—86
Multicentre Phase II, 6-month open label trials in patients with localised prostate cancer eligible for active surveillance, to determine optimal treatment conditions for tumour ablation (using dose, power and light escalation) and to assess the effects of WST-11 mediated VTP
WST-11 (TOOKAD-soluble/padeliporfin)
The technique is well tolerated and reproducible—good efficacy demonstrated when optimal treatment conditions were used
Prostatitis-2; haematuria-1; epididymo-orchitis-1; optic neuropathy-1; urethral stricture-1
PCM301
400 (recruited May 2013)
Multicentre Phase III, open label, randomised controlled study in subjects with low-risk prostate cancer on TRUS-biopsy
Patients randomised to VTP underwent IV administration of WST-11 with 753 nm laser light at 150 mW/cm and 200 J/cm through transperineal interstitial optical fibres with US guidance
WST-11 (200 AS/200 VTP)
Awaited
Awaited
21.6 Treatment Technique
21.6.1 Light Delivery
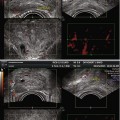
Most photosensitizers absorb light at a specific wavelength which produces maximal photodynamic effect and can be delivered from a laser directed along optical fibres. The use of fibres has allowed treatment of both superficial lesions, and interstitial treatments, as the fibres can be inserted directly to the target organ. The patient is placed in the lithotomy position, under general anaesthetic, in a darkened operating theatre with eye and skin protection. The optical fibres are inserted transperineally using a brachytherapy template on a stepper device, guided with transrectal ultrasound imaging (see Fig. 21.1). Fibres may be either bare ended, with light directly emitted from the end of the fibre, or cylindrical, with a diffusing end. For bare-ended fibres, light is distributed in all directions from the end of the fibre, like a torch or flashlight, and the light dose is measured in J/cm2. The dose from the active length of cylindrical fibres is measured in J/cm, comparable to a ‘strip light’. Bare-ended fibres allow light delivery along a given length at the distal end of the fibre and can be used for superficial treatments using endoscopic access to hollow organs or on the skin itself. Cylindrical diffusers are more commonly used for treatments in solid organs, such as the prostate.
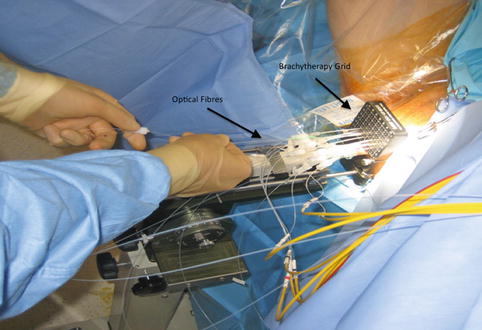

Fig. 21.1
PDT – Brachytherapy grid and fibres labelled
In preclinical studies, optical fibres were also inserted either with an open technique at laparotomy. Although technically feasible for human treatments, open fibre insertion would be inappropriate considering the other available options (transurethral/transperineal). In animal studies, the transurethral approach was associated with urethral strictures and has not been clinically evaluated for use in treatment of prostate cancer (Selman and Keck 1994). In current clinical trials, treatment is delivered using a transperineal template and transrectal ultrasound guidance.
21.6.2 Treatment Planning
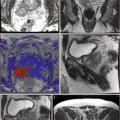
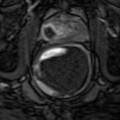
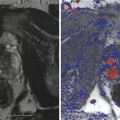
Treatment planning may be either done prior to treatment with a ‘rule-based’ approach or with a ‘real-time’ approach during treatment itself, using feedback from light, drug and oxygen measurements (Moore et al. 2011). With individualised pretreatment planning, account can be made for anatomical variations, with predetermination of the number and position of treatment fibres and light characteristics. The first reported clinical demonstration of patient-specific treatment planning for photodynamic therapy was in the TOOKAD study of recurrent prostate cancer (Davidson et al. 2009). The largest studies of this vascular-targeted PDT for prostate cancer used MR imaging to determine prostate anatomy prior to treatment, with subsequent planning of fibre placement. A retrospective review of patients treated with the water-soluble WST-11 was used to produce a model for treatment planning, with demonstration of correlation between the treatment plan and posttreatment MR imaging (Betrouni et al. 2011). In the TOOKAD study currently underway, a study committee remotely determines the treatment plan, which is sent to the treating centres prior to the scheduled treatment.
Pretreatment planning has some disadvantages, including the anatomical deformation due to the effect of the rectal ultrasound probe and intra-operative swelling of the prostate due to fibre insertion and treatment effect. Even for a pretreatment MR-based plan, it may well be necessary to alter the grid positions and add or exclude light delivery fibres once the prostate is seen under real-time ultrasound guidance.
Canine studies have demonstrated the feasibility of real-time treatment planning modified according to light dose received (Jankun et al. 2005), and it has been reported in a small cohort of patients (Swartling et al. 2010




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree