Fig. 21.1
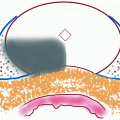
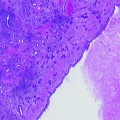
Set-up for VTP procedure. (a) Set-up of the apparatus for vascular-targeted photodynamic therapy. The transrectal ultrasound is in position, with the transperineal template clearly seen. The optical fibres are placed within the hollow plastic needles which are seen protruding from the template. (b) The needles are viewed on ultrasound whilst they are being positioned in the prostate
Preclinical Work with Vascular-Activated Photosensitisers for Prostate Cancer
The first studies using Tookad (WST-09), the vascular-activated photosensitiser that is now in the later stages of clinical development for prostate cancer, were carried out at the Weizmann Institute, Israel, where Avigdor Scherz led the development of the drug from a bacteriochlorophyll derivative. The addition of palladium gave photostability to the drug [7]. Once the in vitro work had established that Tookad resulted in cell death, the first animal studies using implanted gliomas were carried out by Yoram Solamon and his team [8]. This showed that not only was the photosensitiser effective at the local treatment of the implanted tumours but that it was also associated with a greater chance of cure than surgery. It is thought that this may be because of the immune reaction that can occur with PDT and allow sustained antitumour activity [9]. Further work was done assessing the effect of Tookad PDT in a human prostatic small-cell model [10].
When early work had shown promise in the use of Tookad as a treatment for cancer in small-animal models, it was necessary to carry out studies in a larger animal model with prostate anatomy relevant to that in the human. The dog has the prostate structure closest to that seen in the human and is the commonest model used in the evaluation of prostate ablative techniques. The disadvantage of this model is that there is no prostate cancer model in the dog, so most groups use the benign prostate. There have been a few reports of prostate photodynamic therapy used to treat naturally occurring prostate cancer in the dog [11, 12], including one report of a vascular-activated photosensitiser [13].
The canine work with the palladium bacteriopheophorbide photosensitisers used a laparotomy approach to insert the optical fibres [14]. The first report used both superficial illumination and an interstitial approach to deliver light to the prostate; at the same time, light was delivered to the serosal surfaces of the colon and bladder in order to estimate the effect that unintended light delivery in these areas might have in the clinical setting. Temperature monitoring was done during treatment, and a maximum temperature rise of 0.9°C was noted, confirming that the post-procedure effects seen were not due to a thermal laser effect. Sixteen dogs were studied, including one dog given drug only, one dog given the light dose only, and a third control dog receiving neither drug nor light. The drug dose was 2 mg/kg for all the dogs who received it, whilst the light dose varied from 50 to 200 J/cm for interstitial light delivery given using cylindrical diffusers and 100–200 J/cm2 for superficial illumination using a bare-ended fibre.
None of the dogs had urinary retention or any apparent urine symptoms following the procedure.
No effect was seen in any of the control dogs. The treated prostates harvested at 1 week showed acute haemorrhagic necrosis with patchy subcapsular hyperaemia and marked oedema, with the volume of lesion correlating with the light dose given. One prostate was harvested at 4 weeks, when the volume of the lesion was seen to be smaller than for the same dose parameters when harvested at 1 week; in the two prostates harvested at 3 months, significant reduction in gland volume and scarring was seen, suggesting that healing of lesions occurs over time.
Direct illumination at 40 J/cm2 to areas within the bladder showed mild haemorrhage and oedema on histopathological examination, but without evidence of necrosis. The colon showed similar changes at the same direct light dose, whilst a dose of 80 J/cm2 resulted in mucosal ulceration at 1 week, but without perforation. When the effects of a scattered light dose to the colon or bladder, such as might occur from unintended light dose given at the time of light delivery to the prostate, no histopathological effect was seen.
This work then formed the basis of a number of other canine studies, prior to the initiation of a clinical programme. Due to the interest in treating men with recurrent prostate cancer following radiotherapy, one of these studies pretreated dogs with ionising radiation to the prostate prior to WST-09 PDT [15]. Four dogs were given a total of 70 Gy in 20 fractions over 4 weeks. After an interval of 20–23 weeks, WST-09 PDT was given, using a maximum of 1-cm diffuser fibre per lobe, at the same drug dose (2 mg/kg) and light doses of 50, 100, and 200 J/cm. Again, no dogs experienced urinary retention or evidence of urinary symptoms, although traces of blood were seen on urinalysis at 24 h.
The lesion size was seen to correlate with the light dose given, with diameters ranging from 12 to 28, across the spectrum of light doses, with some variation within each light dose group. The length of lesion was longer than the diffuser fibre, particularly at the higher light doses (10–18 mm in length). Histopathological examination of all 4 animals at 1 week showed identical features to the lesions seen in the dogs which had not had previous radiotherapy. Chronic fibrosis, due to the radiotherapy, was clearly distinguishable from the acute PDT effect. Interestingly, even when the urethra was within the treatment zone, no urethral effect was seen. This work formed the basis of the salvage PDT studies carried out in men, with radiorecurrent prostate cancer in Canada, and is discussed in detail in the next section.
Another aspect that was explored in the canine studies was the posttreatment assessment of VTP effects with the use of imaging, in the form of MRI [16]. Five dogs had T2-weighted, dynamic contrast-enhanced and DWI images performed prior to Tookad PDT, and then at 2 days (1 dog), 7 days (5 dogs), and 1 month (1 dog). For reasons that are not clear, a lower drug dose of 1 mg/kg was used in these animals, with light delivery at 50–300 J/cm, using a 1-cm cylindrical diffuser. Four of the prostates were harvested at 1 week, and one at 1 month, and 3-mm step-section whole-mount histopathological correlation with the MR images was performed. It was seen that the gadolinium-enhanced images gave the most accurate correlation with the histopathologically defined volume of effect, and this led to the use of a 1-week contrast-enhanced scan to define the volume of effect in the clinical studies. DCE-MRI showed a central zone of lack of uptake of gadolinium, surrounded by an outer zone of increased enhancement. This effect has also been reported in clinical MR studies following high-intensity focused ultrasound (HIFU) treatment [17]. The WST-09 canine study showed that the central non-enhancing area was an area of acute haemorrhagic necrosis with hyperaemia and marked oedema. The outer ring, seen as enhancing tissue on DCE-MRI, was characterised by atrophic glandular tissue, patchy mononuclear cell infiltration, fibromuscular hyperplasia, neovascularisation, oedema, and dilatated glandular structures.
The histopathological changes seen at 1 month (n = 1) were partial resolution of necrosis with some fibrosis and atrophy, and these were seen as non-enhancing areas on MRI.
A concern in any treatment for prostate cancer is the potential effect on the nerves involved in erections. There had been some suggestions with other photosensitisers that PDT may be “nerve sparing”, either because the drug or light dose may not be able to get into the nerves. This had been noted with both animal studies [18–20] and clinical studies [21]. A study looking at the effect of WST-09 PDT on peripheral nerves in a canine saphenous nerve model did, however, show a dose-dependent effect of PDT on the nerve, with effects seen using direct irradiation at a dose of 100–200 J/cm2 in combination with a drug dose of 1–2 mg/kg [22]. The nerve changes were not seen immediately, but were apparent on both stimulation testing and histological examination at 1 week.
This substantial body of canine work enabled the introduction of WST-09 PDT into the clinical arena. The first studies were carried out in radiorecurrent prostate cancer following definitive radiotherapy, and these will be discussed in detail in the following section.
Tookad Vascular-Targeted Photodynamic Therapy in Men with Radiorecurrent Prostate Cancer
The first-in-man study using WST-09 for prostate cancer was carried out by Trachtenberg and colleagues in men with radiorecurrent prostate cancer in three centres in Canada [23]. Radiorecurrent prostate cancer was suspected on the basis of three consecutive rises in PSA and confirmed on transrectal biopsy. Organ-confined disease status was assigned following digital rectal examination, CT, and bone scanning. The initial part of the study involved a drug dose-escalation phase, where groups of three men were given a drug dose of 0.1, 0.25, 0.5, 1, and 2 mg/kg, respectively. Those in the first phase of study received a fixed light dose of 100 J/cm. The second part of the study, reported separately, involved a light dose-escalation phase for men treated at the 2 mg/kg dose, with escalation to doses of 230 and 360 J/cm [24].
The vascular-targeted photodynamic (VTP) procedure was carried out under general anaesthetic, with the patient in the lithotomy position. Prophylactic antibiotics were given prior to the procedure. A urinary catheter containing a light detector was inserted into the bladder. A transrectal ultrasound was placed in the rectum and secured within a stepper which allowed movement in the x, y, and z axes. After appropriate skin preparation, and with the use of a transperineal template designed for use in prostate brachytherapy, the light delivery catheters were inserted into the prostate. These catheters were again designed for use in prostate brachytherapy and required a track to be made in the prostate using a sharp metal needle. This sharp needle was then removed, and the hollow plastic catheter with a metal inner needle was inserted along the track. Once the positioning of the catheter was seen to be satisfactory on ultrasound, the inner needle was removed. Measurements of the catheter within the prostate allowed an appropriate length of cylindrically diffusing fibre to be selected from the available lengths of 1–4 cm, in 0.5 cm increments. A hydrodissection procedure was carried out to increase the space between the rectum and prostate, with the intention of reducing the light available in the rectum, and so reduce the risk of activation of any drug in the rectum, which could result in a recto-urethral injury.
Optical monitoring fibres were placed in the prostate and rectum, as well as the one in the urinary catheter. Once the planned fibres had been inserted, optical measurements were taken. If the light dose in the rectum was greater than 10% of that in the prostate, then fibres would be removed or repositioned until the rectal fluence fell below this level. Once the fibre positioning was finalised, the drug infusion was begun. The light dose was then given between 6 and 10 min after the end of the infusion. In the first studies, blood samples were taken to assess the peak concentration of the photosensitiser. These showed that the photosensitiser concentration peaked at the end of the infusion and then cleared with a half-life of about 20 min.
Skin sensitivity, previously a significant disadvantage to systemically administered photosensitisers, was formally assessed by Trachtenbergs’ group [25]. Men were kept in dimmed light for 24 h after photosensitiser administration, and skin photosensitivity testing was carried out using a solar simulator directed at small areas of skin, on the back of each patient, at 3-, 12- and 24-h post-PDT. The areas were then assessed for photosensitivity reactions at 24-, 48-, and 72-h post-PDT. Patients were advised to wear dark clothing and wavelength-specific protective eyewear for 7 days after the photosensitiser infusion, although they were discharged from hospital at 24-h post-procedure. No skin response to UV negative light was seen at any time point, for skin exposures of up to 128 J/cm2 and the maximum drug dose of 2 mg/kg. An assessment was also made with UV positive light, which, at a high enough dose, would cause a skin reaction in the absence of a photosensitiser. This was intended to see if the photosensitiser would potentiate the effect of the UV light and cause a greater extent of “sunburn” reaction than would be expected from the light alone. This was not the case at any of the light activation doses or time points tested. This work allowed for the relaxation of the light protection rules in the clinical work which followed so that patients could be discharged on the day of the procedure without restrictive light protection requirements being needed.
The outcomes of the post-radiotherapy studies were reported in a number of different publications. The first report, of the first 24 men, reported the volume of effect as seen on 1-week gadolinium-enhanced MRI, as well as targeted transrectal biopsies to the lesion and serial PSA measurements [23]. They noted that those treated at the lower drug doses of 0.1, 0.25, and 0.5 mg/kg did not show any evident effect on any of the parameters. At both 1 and 2 mg/kg, effects were seen, with all six men in the 2 mg/kg group showing an effect at the highest light dose. No serious adverse events were noted, with only two men not regaining normal urination by day 7. In those men with more than a 20% response on MR criteria, there was a significant decrease in urinary function as determined by the IPSS and Patient-Oriented Prostate Utility Scale at 1 month, but this gradually returned to baseline by 6 months. A drop in blood pressure at 5 min after the infusion of the drug was seen in 12 men, but this responded promptly to fluids and/or vasopressors, and no adverse events related to hypotension were recorded.
Thermal recordings in this first-in-man study confirmed that there was no significant temperature rise during treatment, and so intraprostatic temperature measurements were omitted from further clinical work.
The PSA response was seen to be negligible in those men who had less than a 20% VTP effect on 1 week MRI. In those men who had the highest drug and light dose (six men) and had a greater than 20% effect on 1 week MRI (five men, deemed responders), there was a PSA response where the PSA decreased to negligible levels following the procedure in four men, whilst the fifth man showed a significant but transient PSA response.
The next publication looked specifically at the MR imaging appearance following PDT for men within the light dose-escalation phase [26]. Twenty-five men treated at 2 mg/kg were reported in this study, with three men excluded as they did not complete imaging follow-up, two of whom did not undergo the 6-month biopsy. The imaging analysis was therefore of 25 men, and the biopsy analysis of 26 men. Contrast-enhanced images were taken immediately after (dynamic contrast-enhanced) and at 10 min after the administration of the contrast agent gadopentetate dimeglumine (Magnevist). Confirming the results seen in the canine studies, the treatment effect on T2-weighted imaging was somewhat ill defined at 1 week, and contrast imaging gave the most useful information. A measurement of the percentage of VTP effect was calculated as the volume of non-enhancing tissue outlined from planimetry-based calculations of the region of interest on sequential MR images, divided by the whole prostate volume derived in a similar manner. The effect was noted to be between 0.9% and 80% and varied with the light dose used. The maximal VTP effect was seen at 1 week in the majority of men.
Some of the men showed discontinuous effects which were unexpected, with apparent preservation of tissue between treated areas. Urethral preservation was seen in 10 men, when the treatment plan predicted a urethral effect in 24 of the 25 men. Fifteen men showed a lack of enhancement of the urethra on 1-week MRI—the reasons for the difference in responsiveness of the urethra in some men are not clear, although it may be related to the urethral blood supply.
Irregular treatment margins were noted, with the majority of men (22/25) showing some extraprostatic effects, which were not always contiguous with the intraprostatic effects. Involvement of the puborectalis or levator ani muscles was seen in 22 of 25 men, involvement of the obturator internus in three men, and of the anterior rectal wall in nine men. These extraprostatic changes had resolved in over two-thirds of men by the 6-month scan. Bone marrow changes in the pubic bone were seen in four men. These changes were not visible at 1 week, partially visible at 4 weeks and maximally visible at 6 months. There did not seem to be any association with clinical symptoms with these bone changes.
The rectal wall changes were seen on the 1-week MR scans as loss of enhancement of the outer rectal wall (muscularis propria) in seven men with partial involvement of the mucosa in two men. One of these men developed the clinical symptoms of a fistula, although none was specifically detected on MR imaging or cystoscopy. The symptoms resolved with conservative management. In those men in whom asymptomatic rectal wall involvement was noted on MR imaging at 1 week, these changes had resolved by the scan at 6 months, with associated scarring and reduction in volume of the prostate.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree