A review of pediatric pineal region tumors is provided with emphasis on advanced imaging techniques. The 3 major categories of pineal region tumors include germ cell tumors, pineal parenchymal tumors, and tumors arising from adjacent structures such as tectal astrocytomas. The clinical presentation, biochemical markers, and imaging of these types of tumors are reviewed.
Key points
- •
Diffusion-weighted imaging is a tool to identify hypercellular pineal region tumors, including germ cell tumors and pineoblastomas.
- •
MR spectroscopy can help differentiate germ cell tumors, pineoblastomas, and tectal astrocytomas based on differing metabolic profiles.
- •
Response to therapy of germ cell tumors as well as pineoblastomas can be evaluated using diffusion- and perfusion-weighted imaging.
Introduction
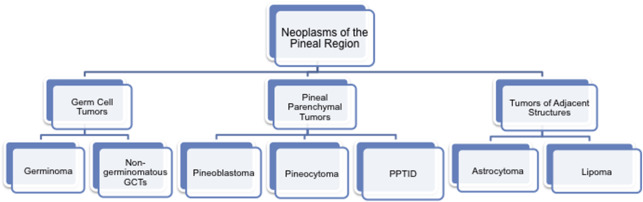
Brain tumors are the second most common malignancy in the pediatric population and the leading cause of death from solid tumors. Neoplasms arising from the pineal region account for approximately 4% of childhood intracranial tumors in the United States, with higher occurrence reported in Asia of up to 9%. These tumors of the pineal region are classified into 3 categories as follows: germ cell tumors, tumors that arise from the pineal parenchyma, and tumors that arise from adjacent structures such as astrocytomas ( Fig. 1 ). Understanding the imaging characteristics, including advanced imaging techniques, in conjunction with the clinical/laboratory findings, can help to differentiate among pineal brain tumors.
The clinical presentation of children with pineal region lesions is variable and is often related to mass effect on the adjacent structures. The clinical signs and symptoms can include precocious puberty, Perinaud syndrome secondary to mass effect on the tectum, headache, and nausea/vomiting. In addition to the clinical presentation, it is important to recognize that some of the tumors arising in the pineal region have associated oncologic markers that can be identified with both serologic and cerebrospinal fluid (CSF) sampling, including alpha fetoprotein, beta human chorionic gonadotropin, and placental alkaline phosphatase ( Table 1 ).
| Type of GCT | AFP | B-hCG | PLAP |
|---|---|---|---|
| Pure germinoma | − | − | + or − |
| Syncytiotrophoblastic germinoma | − | + | + or − |
| Mature teratoma | − | − | − |
| Immature teratoma | + or − | + or − | − |
| Choriocarcinoma | − | + | + or − |
| Yolk sac | + | − | + or − |
| Embryonal | − | − | + |
| Mixed GCT | + or − | + or − | + or − |
In this article, we review the normal anatomy of the pineal region, discuss the differential diagnosis of pineal region masses, and present the imaging findings for these tumors with an emphasis on advanced imaging techniques, such as diffusion, perfusion, and spectroscopy.
Introduction
Brain tumors are the second most common malignancy in the pediatric population and the leading cause of death from solid tumors. Neoplasms arising from the pineal region account for approximately 4% of childhood intracranial tumors in the United States, with higher occurrence reported in Asia of up to 9%. These tumors of the pineal region are classified into 3 categories as follows: germ cell tumors, tumors that arise from the pineal parenchyma, and tumors that arise from adjacent structures such as astrocytomas ( Fig. 1 ). Understanding the imaging characteristics, including advanced imaging techniques, in conjunction with the clinical/laboratory findings, can help to differentiate among pineal brain tumors.
The clinical presentation of children with pineal region lesions is variable and is often related to mass effect on the adjacent structures. The clinical signs and symptoms can include precocious puberty, Perinaud syndrome secondary to mass effect on the tectum, headache, and nausea/vomiting. In addition to the clinical presentation, it is important to recognize that some of the tumors arising in the pineal region have associated oncologic markers that can be identified with both serologic and cerebrospinal fluid (CSF) sampling, including alpha fetoprotein, beta human chorionic gonadotropin, and placental alkaline phosphatase ( Table 1 ).
| Type of GCT | AFP | B-hCG | PLAP |
|---|---|---|---|
| Pure germinoma | − | − | + or − |
| Syncytiotrophoblastic germinoma | − | + | + or − |
| Mature teratoma | − | − | − |
| Immature teratoma | + or − | + or − | − |
| Choriocarcinoma | − | + | + or − |
| Yolk sac | + | − | + or − |
| Embryonal | − | − | + |
| Mixed GCT | + or − | + or − | + or − |
In this article, we review the normal anatomy of the pineal region, discuss the differential diagnosis of pineal region masses, and present the imaging findings for these tumors with an emphasis on advanced imaging techniques, such as diffusion, perfusion, and spectroscopy.
Normal anatomy and imaging technique
The pineal gland is a small structure located midline, above the tentorium and superior colliculi and below the splenium of the corpus callosum and the vein of Galen, attached to the posterior border of the third ventricle. The pineal region includes the pineal gland, the surrounding cisterns of the quadrigeminal plate and velum interpositum, posterior third ventricle, and adjacent tissue of the brainstem, thalami, and splenium of the corpus callosum as seen in Fig. 2 . The pineal gland normally measures less than 8 mm in greatest dimension and can be commonly calcified. However, calcification is rare under the age of 10 years.
Imaging protocols for the evaluation of pineal region masses include both conventional and advanced MR techniques. Although computed tomography (CT) may be the method most commonly used for the initial presentation of the patient, MR is the study of choice for the evaluation and characterization of brain tumors, including pineal region masses. A summary of both conventional and advanced imaging sequences is presented in Table 2 . Conventional sequences, which can be obtained on 1.5 or 3 T magnets, include T1-weighted, T2-weighted, and Fluid-attenuated inversion recovery (FLAIR) imaging. These sequences should be acquired in multiple planes. For midline lesions such as pineal region masses, sagittal plane is a necessity. Sagittal T1 sequence should preferably be acquired using a 3-dimensional gradient echo sequence to generate reformations of additional planes for detailed analysis. Intravenous gadolinium compounds are used to assess the degree of permeability of the blood–tumor barrier and to assess for CSF dissemination of tumor.
| Sequence | GCT | Pineoblastoma | Astrocytoma |
|---|---|---|---|
| T1 | Isointense/hypointnese to GM | Heterogeneous | Hypointense to GM |
| T2 | Isointense/hypointense or slightly hyperintense to GM | Heterogeneous | Hyperintense to GM |
| GRE | ± Susceptibility secondary to hemorrhage | ± Susceptibility secondary to hemorrhage | ± Susceptibility secondary to hemorrhage |
| Contrast | Avid enhancement, heterogeneous | Moderate heterogeneous (may not enhance) | ± Enhancement |
| Diffusion-weighted imaging | Restricted | Restricted | Restricted in areas of higher grade (III/IV) |
| Dynamic susceptibility contrast perfusion | ↑ CBV (may be related to T2 effect) | Limited data | ↑ CBV in areas of higher grade (III/IV) |
| Arterial spin labeling perfusion | Limited data | Limited data | ↑ CBF in areas of higher grade (III/IV) |
| MRS | ↑ Cho, ↓ NAA, ± taurine | ↑ Cho, ↓ NAA, ± taurine (myoinositol may be seen) | ↑ Cho, ↓ NAA, absent taurine |
Advanced imaging techniques such as diffusion-weighted imaging (DWI), perfusion imaging, and spectroscopy are used to assess tumor cellularity, vascular density, and metabolic profile, respectively. DWI is used routinely for the evaluation of brain tumors to distinguish hypercellular from hypocellular tumor. Perfusion imaging can be acquired both with gadolinium intravenous contrast agents as well as without gadolinium using arterial spin labeling. The techniques used for perfusion imaging include T2*-weighted dynamic susceptibility, arterial spin labeling, and T1-weighted dynamic contrast-enhanced imaging. Perfusion imaging, both dynamic contrast-enhanced perfusion techniques using exogenous tracer agents such as gadolinium-based contrast or endogenous agents such as magnetically labeled blood, reflect tumor vascularity as well as tumor grade. MR spectroscopy (MRS) is also a technique that has been shown to differentiate lower from higher grade tumors. The metabolic profile, including differences in key metabolites such as NAA, choline, myoinositol, and the presence of lipids and lactate, can help to narrow the differential diagnosis for pineal region tumors.
Imaging findings and pathology
Germ Cell Tumors
The most common pineal region neoplasms are malignant germ cell tumors, often occurring in adolescent male patients. These lesions account for greater than one-half of the pineal region neoplasms. The World Health Organization (WHO) classifies germ cell tumors as germinomas and nongerminomatous germ cell tumors. The nongerminomatous germ cell tumors include teratomas, embryonal carcinoma, yolk sac tumor, choriocarcinoma, and mixed germ cell tumors.
Germ cell tumors are derivatives of primordial ectoderm, mesoderm, or endoderm, with each tumor subtype representing the neoplastic correlate of a distinct stage of embryonic development. Within the germ cell tumor group, there is a distinction between neoplasms that form embryonic tissue and those that form extraembryonic tissue. Germinomas are the most common type of pineal tumors, accounting for more than 60% of germ cell tumors and approximately 40% of pineal region neoplasms. These types of tumors affect male patients 2 to 17 times more often than female patients and are found predominantly in the pineal region, with approximately 20% of germinomas occurring outside of the pineal region in the suprasellar region or basal ganglia. Germinomas may also compromise the corpus callosum as well as cranial nerves, mostly the trigeminal and optic nerves. Germinomas are not encapsulated tumors and therefore can invade adjacent structures of the brain and spread along the surface of the brain or through CSF.
Germ cell tumors are diagnosed by a combination of serum and CSF markers as well as imaging. These tumors result in elevated serum and CSF oncoproteins such as alphafetoprotein, beta human chorionic gonadotropin, and placental alkaline phosphatase (see Table 1 ). Germinomas are divided into 2 subgroups: pure germinomas and germinomas with syncytiotrophoblastic cells, which have a higher recurrence rate and decreased long-term survival, with increased CSF beta human chorionic gonadotropin. Treatment regimens include chemotherapy, radiation, or a combination of both with a favorable prognosis and 5-year survival of at least 90%.
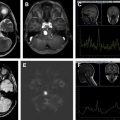
On imaging, germinomas can have a heterogeneous appearance, often seen as a solid or solid/cystic mass with increased attenuation on CT. Often, the relationship between the tumor and the pattern of calcification within the pineal gland can help to distinguish germinomas from pineal parenchymal tumors, such as pineoblastomas. Calcification is typically engulfed by the tumor in germinomas, whereas in pineoblastomas, it is scattered in the periphery of the tumor ( Fig. 3 ). Other characteristic features differentiating germinomas from pineoblastomas are listed in Table 3 , including multiplicity. Additional conventional imaging characteristics of germinomas include decreased T2 signal as compared with gray matter and heterogeneous enhancement.