Abstract
The rising rates of cesarean delivery in recent years have led to an increase in the diagnosis of placenta accreta, increta, and percreta. A high index of suspicion and prenatal imaging are critical for identifying patients at risk and planning for delivery. A diagnosis of placenta accreta should be considered in all women with any of the following: prior cesarean delivery, placenta previa or low-lying placenta and a history of prior uterine surgery, Asherman syndrome, endometrial ablation, or pelvic radiation. Women with risk factors should undergo a targeted US assessment for possible placenta accreta in the midtrimester. Transabdominal and transvaginal US can be further enhanced by use of Doppler imaging and potentially three-dimensional (3D) imaging. Magnetic resonance imaging (MRI) may be used adjunctively in certain conditions. When accreta is suspected, the patient should be counseled about potential morbidity and the possibility of hysterectomy. Delivery by a multidisciplinary team at a high-volume center or accreta center of excellence is recommended.
Keywords
placenta accreta, morbidly adherent placenta, abnormally invasive placentation, ultrasound placenta, accreta center of excellence
Introduction
The rising rates of cesarean delivery in recent years have led to an increase in the diagnosis of placenta accreta, increta, and percreta. Also referred to as morbidly adherent placenta or abnormally invasive placentation, this spectrum of conditions refers to excessive invasion of the placenta into the uterus. Placenta accreta is associated with significant maternal morbidity and a mortality rate of up to 7%. However, delivery in a tertiary referral center by a multidisciplinary team, consistent with the proposed criteria for an accreta “center of excellence,” has been shown to attenuate these risks. A high index of suspicion and prenatal imaging are critical for identifying patients at risk and planning for delivery.
Disease
Definition
Placenta accreta specifically indicates trophoblastic invasion beyond the Nitabuch fibrinoid layer but not into the myometrium. Placenta increta describes invasion into the myometrium. Placenta percreta indicates invasion beyond the uterine serosa, which may involve adjacent organs such as the bladder or vessels. This chapter uses the term placenta accreta to describe all three conditions.
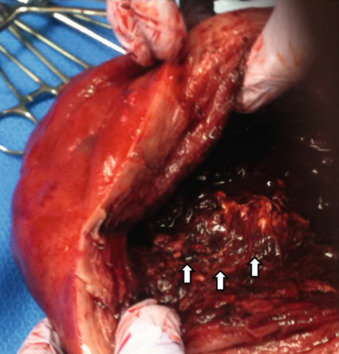
While placenta accreta may be suspected prenatally, the clinical diagnosis is made when either part or the entire placenta fails to separate from the uterus at the time of delivery, often resulting in massive hemorrhage requiring hysterectomy. Fig. 97.1 shows a hysterectomy specimen with placental tissue adherent to the myometrium after attempted removal of the placenta in a case where diagnosis was not suspected prenatally. Pathologic examination of the surgical specimen is the gold standard to confirm the diagnosis by histologic demonstration of abnormal trophoblastic invasion.
Prevalence and Epidemiology
Placenta accreta is reported to complicate 1 : 533 to 1 : 2500 deliveries; this represents a 10-fold increase over the last 50 years. The major risk factors are prior cesarean deliveries and the presence of placenta previa in the current pregnancy. In the presence of placenta previa, the risk of invasive placentation increases with the number of previous cesarean deliveries, reaching 50% to 67% with four or more previous cesarean deliveries. Additional risk factors include advanced maternal age, smoking, multiparity, previous curettage or myomectomy, Asherman syndrome, prior endometrial ablation, a history of pelvic radiation, and submucosal fibroids. Among cases of invasive placentation, the majority (75%) are consistent with a histologic diagnosis of accreta, whereas diagnoses of increta (18%) or percreta (7%) are less common.
Etiology and Pathophysiology
Defects of the decidua basalis and excessive trophoblast invasion secondary to failed reconstitution of the endometrium after prior injury are considered to be underlying predisposing factors for invasive placental implantation. Histologic confirmation of placenta accreta usually involves demonstration of trophoblasts in direct contact with the myometrium, invading the myometrium, or extending into or beyond the uterine serosa.
Manifestations of Disease
Clinical Presentation
The most important step in diagnosis is to have a high index of suspicion for a diagnosis of placenta accreta in patients with risk factors. A history of cesarean delivery or prior uterine surgery, particularly in women who also have a diagnosis of placenta previa or low-lying placenta in the current pregnancy, should prompt a thorough evaluation for evidence of placenta accreta. Patients may remain asymptomatic during the prenatal period, or, if there is placenta previa or low-lying placenta, they may present with bleeding. There have been a few reports of spontaneous uterine rupture and subsequent intraabdominal hemorrhage in the second half of pregnancy and cases of placenta accreta manifesting with excessive hemorrhage at the time of dilatation and evacuation in the second trimester. There are no prenatal symptoms that are specific to the diagnosis of invasive placentation.
Abnormal placental invasion may be apparent at the time of laparotomy for cesarean delivery: the placental mass may be seen distorting the lower uterine segment, sometimes with engorged and abnormal-appearing vasculature, and can extend into the bladder or toward the pelvic sidewalls. However, in some cases, placenta accreta may not be suspected until difficulties are encountered when separating the placenta in the third stage of labor or when heavy bleeding occurs from the implantation site (see Fig. 97.1 ). Women with placenta accreta experience higher estimated blood loss, with an average loss of 3000 to 5000 mL, and require more transfusions than women with previa who do not have accreta. Massive bleeding may require hysterectomy or may result in surgical injury to adjacent organs, acute respiratory distress syndrome, renal failure, disseminated intravascular coagulation, or death.
Imaging Technique and Findings
Ultrasound.
All women with a prior cesarean delivery, placenta previa or a low-lying placenta, prior uterine surgery or a history of Asherman syndrome, prior endometrial ablation, or pelvic radiation should be assessed for possible placenta accreta in the midtrimester. Ordering clinicians should communicate these risks to imagers to ensure that a thorough assessment is performed. Transabdominal and transvaginal ultrasound (US) can be further enhanced by use of Doppler imaging and potentially three-dimensional (3D) imaging. Between careful imaging and evaluation of risk factors, obstetricians can identify patients who will benefit from referral to an accreta center of excellence that is equipped to provide preoperative planning and the necessary intraoperative services.
In the first trimester, US may demonstrate a gestational sac located in the lower uterine segment. A retrospective cohort study of 94,230 patients, 20 of whom were ultimately diagnosed with placenta accreta at the time of delivery, identified seven patients with first-trimester imaging at 10 weeks’ gestation or before. The gestational sac was located in the lower uterine segment in six of seven patients, as opposed to the fundal location in which it is expected to be found before 11 weeks’ gestation. Cesarean scar ectopic pregnancy refers to implantation of the gestational sac at the location of a prior hysterotomy and differs from placenta accreta in that the gestational sac is completely surrounded by myometrium and/or scar tissue, separate from the endometrial cavity. However, growth of trophoblastic tissue may occur in the direction of the cervix and uterine cavity, or toward the bladder and abdominal cavity, eventually resulting in either placenta accreta or abdominal pregnancy. Timor-Tritsch et al. described 60 patients with cesarean scar pregnancies, nine of whom elected expectant management and had outcomes available. Eight patients eventually required hysterectomy: three following cesarean delivery of live-born offspring and five after second-trimester complications including uterine rupture (three), uterine dehiscence (one), and bulging membranes (one). One patient underwent cesarean delivery at 36 weeks and was subsequently lost to follow-up. Sonographically, cesarean scar ectopic pregnancy is differentiated from an incomplete abortion by demonstration of an empty uterine cavity and cervical canal, a gestational sac in the anterior aspect of the uterine isthmus, a defect in the myometrial tissue posterior to the bladder, and circumferential vascular flow around the gestational sac on Doppler imaging.
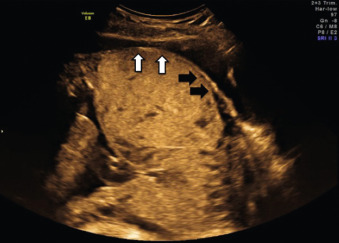
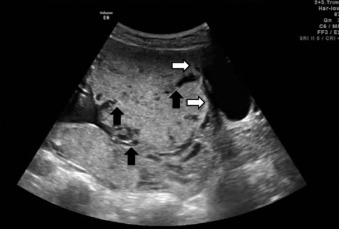
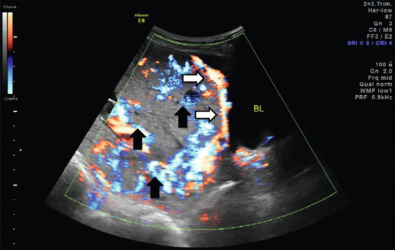
US findings suggestive of placenta accreta in the second and third trimesters include: (1) loss of the retroplacental “clear space,” the hypoechoic border between the placenta and myometrium ( Fig. 97.2 ), (2) bulging of the placenta into the posterior aspect of the bladder because of increased vascularity at the interface with the bladder ( Figs. 97.3 and 97.4 ), and (3) multiple vascular lacunae within the placenta, which appear as irregular anechoic spaces with a “moth-eaten” or “Swiss cheese” appearance and may demonstrate turbulent Doppler flow (see Figs. 97.3 and 97.4 ). Several series have reported on the test characteristics of individual sonographic markers as presented in Table 97.1 . Lacunae are reported to be the single most predictive sonographic marker, with a sensitivity of 79% at 15 to 20 weeks’ gestation and 93% at 15 to 40 weeks’ gestation. Some have reported that the higher the number of lacunae, the higher the risk of placenta accreta. Loss of the retroplacental hypoechoic space has been reported to be responsible for the most false-positive diagnoses. Color flow Doppler US may provide another means of visualizing abnormal vasculature suggestive of invasive placentation. Sensitivity of 82% to 100% and specificity of 72% to 100% have been reported in some series, although these retrospective studies may be limited by case selection bias and single technicians with particular experience with this technique. A technique for estimating the largest confluent area of 3D power Doppler signal at the uteroplacental interface has also been described. The calculated area of confluence (cm 2 ) was reported to provide a quantitative means for the diagnosis of placenta accreta and for determining the severity of invasion. While promising, such calculations should be subject to further validation before being introduced into routine clinical practice.
| Sensitivity % | Specificity % | PPV % | NPV % | |
|---|---|---|---|---|
| Loss of retroplacental clear space | 73–100 | 35–81 | 14–57 | 97–100 |
| Loss of boundary between bladder and placenta | 11–70 | 99–100 | 75–100 | 88–92 |
| Lacunae | 73–100 | 28–97 | 21–94 | 88–100 |
| Turbulent blood flow on color Doppler | 89–100 | 94–100 | 80–100 | 97–100 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






