Pleura, Chest Wall, Diaphragm, and Miscellaneous Chest Disorders
Jeffrey S. Klein
Jimmy S. Ghostine
Pleura
Anatomy, Physiology, and Pathophysiology
The pleura is a serous membrane subdivided into visceral pleura, which covers the lung and forms the interlobar fissures, and parietal pleura, which lines the mediastinum, diaphragm, and thoracic cage. Both the visceral and parietal pleurae consist of a single layer of mesothelial cells and their basement membrane, and a dense sheet of irregular connective with varying ratios of collagen to elastin (1). The potential space between the visceral and parietal pleura is the pleural space. The parietal and visceral pleurae meet at the hila and form a thin double-layered fold at the medial lung base inferior to the inferior pulmonary veins termed the pulmonary ligament (see Fig. 12.8). A small amount of fluid totaling 2 to 5 mL is normally present in the pleural space to serve as a lubricant that allows smooth gliding of the visceral pleura along the parietal pleura during breathing. The volume of fluid within the pleural space is the result of a dynamic equilibrium between formation and resorption (2). The formation of pleural fluid follows Starling’s law and depends upon hydrostatic and oncotic forces in both the systemic capillaries of the parietal pleura and the pleural space (1). Under normal conditions, pleural fluid is formed by filtration from systemic capillaries in the parietal pleura and resorbed via the parietal pleural lymphatics. (Fig. 19.1).
The radiologically detectable manifestations of pleural diseases are limited and include effusion, thickening, and calcification (3).
Pleural Effusion
Pleural effusions form when an imbalance occurs between formation and reabsorption (Table 19.1). Pleural effusions may be classified by their gross appearance (bloody, chylous, purulent, serous), the underlying disease process (Table 19.2), or by the pathophysiology of abnormal pleural fluid formation (i.e., transudative versus exudative) (Tables 19.1 and 19.3). This latter differentiation is made by measuring the protein, lactic acid dehydrogenase (LDH), and glucose concentration of the pleural fluid obtained by thoracentesis (Table 19.3).
Specific Causes of Pleural Effusion
Congestive heart failure is the most common condition to produce a transudative pleural effusion. The effusions are typically bilateral and larger on the right (4). An isolated right effusion is twice as common as an isolated left effusion.
Parapneumonic Effusion and Empyema. A parapneumonic effusion is defined as an effusion associated with pneumonia. Peripheral parenchymal infection produces an exudative pleural effusion by causing visceral pleural inflammation that increases pleural capillary permeability. Inflammatory thickening of the pleural membranes with lymphatic obstruction may also be a contributing factor. Empyema results when the parenchymal infection extends into the pleural space. Parenchymal infections that typically result in empyema formation are bacterial pneumonia, septic emboli, and lung abscess, whereas fungal, viral, and parasitic infections are uncommon causes. Less commonly, infection may extend into the pleural space from the spine, mediastinum, and chest wall.
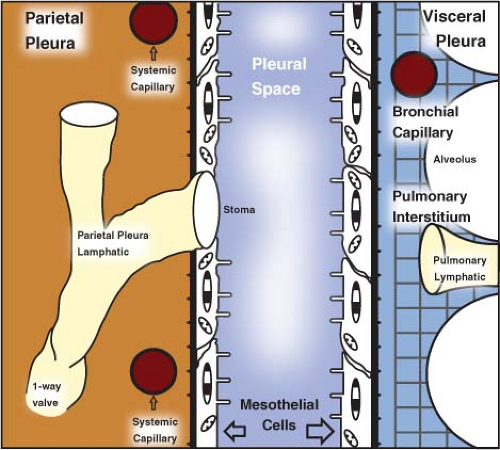 Figure 19.1. Normal Pleural Physiology. (Modified from Miserocchi G. Physiology and pathophysiology of pleural fluid turnover. Eur Respir J 1997;10:219–225.) |
Forty percent of bacterial pneumonias have an associated pleural effusion. Staphylococcus aureus and gram-negative pneumonias are the most common cause of parapneumonic effusion and empyema. The natural history of parapneumonic effusions may be divided into three stages (5,6,7). Stage 1 is an exudative stage; visceral pleural inflammation causes increased capillary permeability and pleural fluid accumulation. Most of these sterile exudative effusions resolve with appropriate antibiotic therapy. A stage 2 parapneumonic effusion is a fibrinopurulent pleural fluid collection containing bacteria and neutrophils. Fibrin deposition on the visceral and parietal pleura impairs fluid resorption and produces loculations. If the infection is not treated, the loculations will impair attempts at closed pleural fluid drainage. A stage 3 parapneumonic effusion develops 2 to 3 weeks after initial pleural fluid formation and is characterized by the ingrowth of fibroblasts over the pleura, which produces pleural fibrosis and entraps the lung. Dystrophic calcification of the pleura may develop following resolution of the pleural infection. Tuberculous pleural effusion or empyema resulting from the rupture of subpleural caseating granulomas may complicate pulmonary infection or occur as the primary manifestation of disease. Effusions in tuberculosis (TB) are more common in young adults with pulmonary disease and in HIV-positive individuals with severe immunodeficiency. The pleural fluid is characteristically straw colored, with greater than 70% lymphocytes and a low glucose concentration.
Table 19.1 Mechanisms of Abnormal Pleural Fluid Formation | ||
|---|---|---|
|
Table 19.2 Etiology of Pleural Effusions | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Table 19.3 Characterization of Pleural Effusions | ||||||
|---|---|---|---|---|---|---|
| ||||||
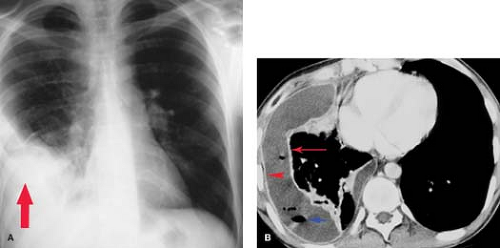
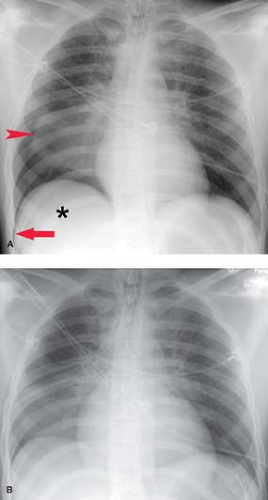
Radiographically, empyema most often appears as a loculated pleural fluid collection. On CT, it is elliptic in shape and is seen most often within the posterior (costal pleura) and inferior (subpulmonic) pleural space. The collection conforms to and maintains a broad area of contact with the chest wall (Fig. 19.2). The distinction of empyema from peripheral lung abscess has important therapeutic implications; empyemas require external drainage, whereas lung abscesses usually respond to postural drainage and antibiotic therapy. Contrast-enhanced chest CT is most useful in making this distinction (Table 19.4) (8). Detection of an empyema may be difficult when there is extensive parenchymal consolidation. In these cases, CT and US are useful in detecting parapneumonic fluid collections and guiding diagnostic thoracentesis and pleural drainage. Findings on CT that are fairly specific for the presence of an exudative pleural effusion include thickening and enhancement of the parietal pleura, the presence of loculations, and the detection of discrete soft tissue lesions along the parietal pleura outlined by low-attenuation pleural fluid. Hemorrhagic effusions can occasionally be recognized on CT by their intrinsic high attenuation or the presence of a fluid–fluid level caused by dependent cellular blood elements.
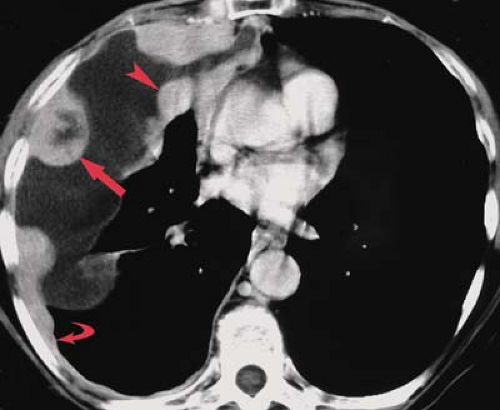
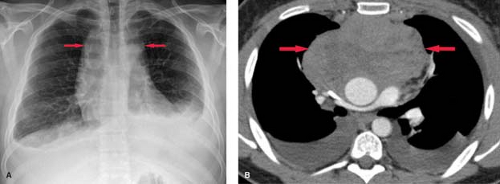
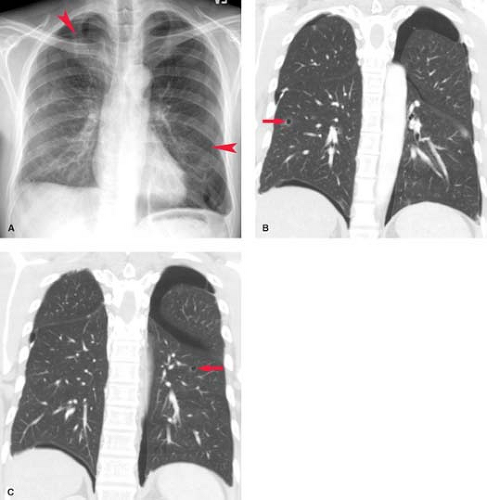
Neoplasms. Pleural effusion may be seen with benign or malignant intrathoracic tumors. The tumors most commonly associated with pleural effusion are, in order of frequency, lung carcinoma, breast carcinoma, pelvic tumors, gastric carcinoma, and lymphoma. Pleural fluid may result from pleural involvement by tumor or from lymphatic obstruction anywhere from the parietal pleura to the mediastinal nodes. The effusions are exudative and may be bloody. Demonstration of malignant cells on cytologic examination of pleural fluid obtained at thoracentesis is necessary for the diagnosis of a malignant effusion. Image-guided closed or thoracoscopic biopsy is reserved for patients with negative cytologic examination. Clues to the presence of a malignant pleural effusion include smooth or nodular pleural thickening, mediastinal or hilar lymph node enlargement or mass, and solitary or multiple parenchymal nodules. CT is useful in demonstrating pleural masses or underlying parenchymal lesions in those with large effusions (Fig. 19.3).
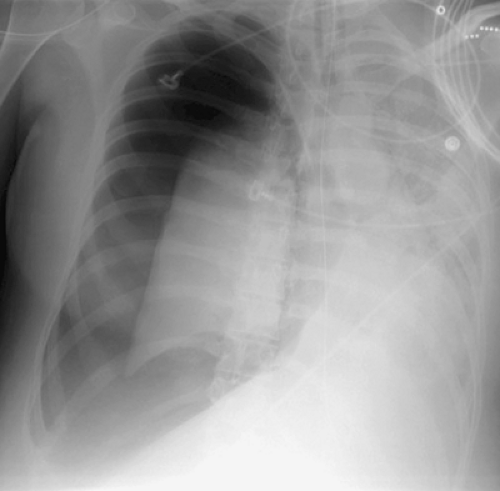
Trauma. Blunt or penetrating trauma to the chest, including iatrogenic trauma from thoracotomy, thoracostomy, or placement of central venous catheters, may result in a hemothorax. Hemothorax results from laceration of vessels within the lung, mediastinum, chest wall, or diaphragm. Intrapleural blood coagulates rapidly, and septations form early. In some individuals, pleural motion causes defibrination, which lyses the clotted blood. In the acute setting, pleural fluid of high
CT attenuation (>80 H) may be seen (Fig. 19.4); associated rib fractures or subcutaneous emphysema should suggest the diagnosis. An acute hemothorax is treated with thoracostomy tube drainage, whereas thoracotomy is generally reserved for persistent bleeding or hypotension.
CT attenuation (>80 H) may be seen (Fig. 19.4); associated rib fractures or subcutaneous emphysema should suggest the diagnosis. An acute hemothorax is treated with thoracostomy tube drainage, whereas thoracotomy is generally reserved for persistent bleeding or hypotension.
Table 19.4 Empyema Versus Lung Abscess on Ct | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Esophageal perforation from prolonged vomiting (Boerhaave syndrome) or as a complication of esophageal dilatation may produce a pleural effusion, most commonly on the left side.
Extravascular placement of a central line can result in a hydrothorax when intravenous solution is inadvertently infused into the pleural or extrapleural space.
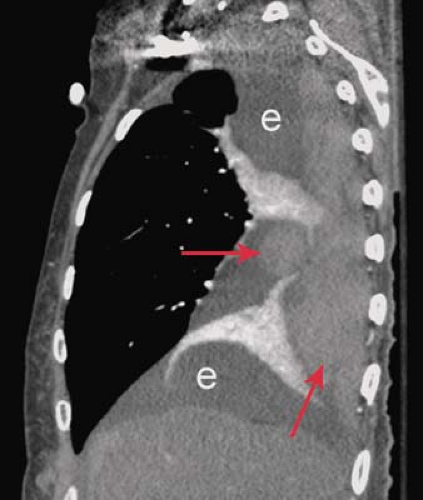
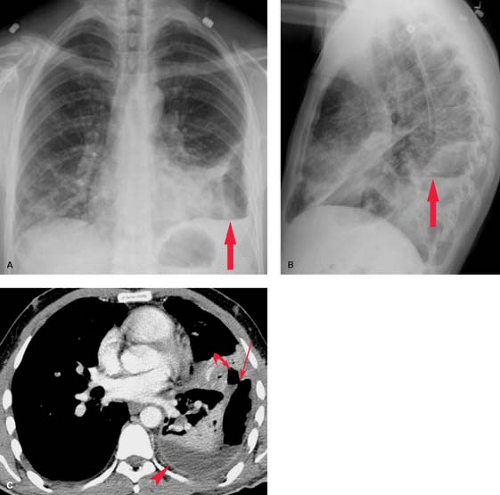
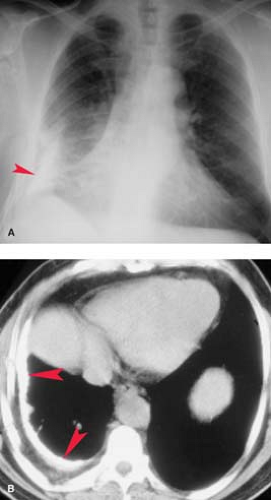
Collagen Vascular and Autoimmune Disease. Systemic lupus erythematosus has a reported incidence of pleural effusions ranging from 33% to 74% (Fig. 19.5). These exudative effusions are a result of pleural inflammation; patients often present with pleuritic chest pain. In some cases, the nephrotic syndrome associated with systemic lupus erythematosus may produce transudative effusions. Cardiomegaly is a common associated radiographic finding and may be caused by pericardial effusion, hypertension, renal failure, or lupus-associated endocarditis or myocarditis. Pleural effusion is the most common intrathoracic manifestation of rheumatoid arthritis and is most frequently seen in male patients following the onset of joint disease. The effusions occur independent of pulmonary parenchymal involvement, but may develop following intrapleural rupture of peripheral rheumatoid nodules. The effusions of rheumatoid arthritis are exudative, with lymphocytosis, low glucose concentration, and low pH (<7.2). Rheumatoid effusions may persist unchanged for years. Autoimmune syndromes producing pleural and pericardial effusions have been described following myocardial infarction (Dressler syndrome) or cardiac surgery (postpericardiotomy syndrome). Both are characterized by fever, pleuritis, pneumonitis, and pericarditis developing within days to weeks of the precipitating event. The radiographic findings include enlargement of the cardiac silhouette, pleural effusions, and parenchymal airspace opacities. A serosanguineous exudative pleural effusion is seen in over 80% of patients. Treatment with nonsteroidal anti-inflammatory drugs usually results in symptomatic and radiographic improvement.
Abdominal Disease. Radioisotope studies have demonstrated that peritoneal fluid may enter the pleural space via transdiaphragmatic lymphatic channels or through defects in the diaphragm. The lymphatic channels are larger on the right side, accounting for the higher incidence of right-sided effusions associated with ascites or liver failure (hepatic hydrothorax).
Pancreatitis. Acute or chronic pancreatitis can cause pleural effusions that are most often left-sided because of the proximity of the pancreatic tail to the left hemidiaphragm. The effusion associated with acute pancreatitis is typically exudative and may be bloody. Pleural effusion from chronic pancreatitis
may cause pleuritic chest pain and shortness of breath. Rupture of the pancreatic duct can lead to a pancreaticopleural fistula. A high amylase concentration in the pleural fluid should suggest the pancreas as the etiology of the effusion, although elevated amylase may be seen in pleural effusions caused by malignancy or esophageal perforation.
may cause pleuritic chest pain and shortness of breath. Rupture of the pancreatic duct can lead to a pancreaticopleural fistula. A high amylase concentration in the pleural fluid should suggest the pancreas as the etiology of the effusion, although elevated amylase may be seen in pleural effusions caused by malignancy or esophageal perforation.
Subphrenic abscess complicating abdominal surgery or perforation of a hollow viscus can cause diaphragmatic paresis, basilar atelectasis, and pleural effusion. Patients with a pleural effusion associated with upper abdominal pain, fever, and leukocytosis should have CT or US examination and when applicable, percutaneous catheter drainage of the abscess.
Pelvic Tumors. An association between benign pleural effusions and pelvic tumors has long been recognized. First described with ovarian fibroma (Meigs syndrome), a number of pelvic and abdominal tumors, including pancreatic and ovarian malignancy, lymphoma, and uterine leiomyomas, have been found to cause pleural effusion. The effusions in Meigs syndrome are usually transudative and resolve after removal of the pelvic tumor.
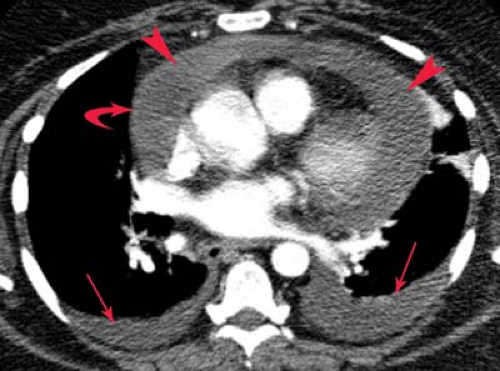
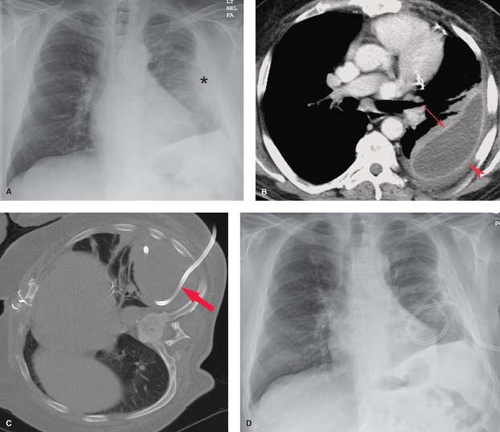
Chylothorax is a pleural collection containing triglycerides in the form of chylomicrons resulting from extravasation of thoracic duct contents secondary to malignancy, iatrogenic trauma, or TB (Fig. 19.6). The thoracic duct originates from the cisterna chyli at the level of the first lumbar vertebra and ascends along the right paravertebral space, entering the thorax via the aortic hiatus. The duct crosses from right to left at the level of the sixth thoracic vertebra to lie alongside the upper esophagus. A knowledge of this anatomy is useful, as disruption of the upper duct caused by direct trauma or obstruction with rupture produces a left chylothorax, whereas injury to the lower intrathoracic duct produces a right chylothorax. At the level of the left subclavian artery, the duct arches anteriorly to empty into the confluence of the left internal jugular and subclavian veins. The radiographic appearance is indistinguishable on plain radiographs and CT from other causes of free-flowing effusions. The diagnosis is confirmed by triglyceride levels exceeding 110 mg/dL in the pleural fluid.
Pulmonary Embolism. Infarction complicating pulmonary embolism is a well-recognized cause of pleural effusion. The effusion may be associated with elevation of the ipsilateral diaphragm and peripheral wedge-shaped opacities (Hampton hump). The pleural effusion is typically a small, unilateral, serosanguineous exudate.
Drugs may cause pleural effusions as a result of pleural inflammation (methysergide) or by producing a lupus-like syndrome (phenytoin, isoniazid, hydralazine, procainamide). Nitrofurantoin has been associated with an immunologic reaction that causes pleuropulmonary disease with eosinophilia.
Management of Pleural Effusion. Transudative pleural effusions are managed by treatment of the underlying disorder because the pleura is intrinsically normal in these diseases. Management of parapneumonic effusions is best guided by evaluation of the likelihood that the effusion, if not drained, would result in prolonged hospitalization, pleural fibrosis with resultant respiratory impairment, local spread of infection, or death. This likelihood is based on the anatomy, bacteriology, and chemistry (i.e., ABCs) of the fluid collection. In general, larger, loculated collections with positive gram stains or cultures and pH less than 7.20 are associated with a moderate to high risk for poor outcome as detailed above and should be drained if possible (5). The choice of drainage procedure depends on various factors, including patient age and underlying condition, length of illness, and access to image-guided therapy and thoracoscopy. Although intrapleural fibrinolytic therapy with tissue plasminogen activator will help a certain subset of patients with complex parapneumonic effusions (Fig. 19.7), some will require open pleural drainage by video-assisted thoracoscopic surgery (VATS) or thoracotomy with decortication. In contrast, malignant pleural effusions most often require closed drainage and pleural sclerosis, with talc being the current agent of choice. Trials of other pleurodesis agents have not shown superiority while being marred by higher cost. It is notable that talc pleurodesis can cause FDG-18 PET positive nodularity, which is a source of false-negative PET evaluations. Some patients may benefit from VATS drainage and sclerosis. Select patients can be managed as outpatients with indwelling silastic catheters (e.g., PleurX™catheter, CareFusion Corp, San Diego, CA), which allow intermittent patient-directed drainage of fluid. Patients with chylothorax secondary to lymphoma or TB require therapy directed at this underlying, whereas patients with traumatic disruption of the thoracic duct often require surgical ligation of the duct.
Patients with pleural effusions from trauma, pulmonary embolism, autoimmune disorders, and drug reactions often require no specific therapy. Exceptions include the postpericardiotomy
or post-MI patients (Dressler syndrome), who are treated with nonsteroidal anti-inflammatory agents, and patients with large hemothoraces requiring large bore tube drainage to prevent pleural fibrosis and lung entrapment.
or post-MI patients (Dressler syndrome), who are treated with nonsteroidal anti-inflammatory agents, and patients with large hemothoraces requiring large bore tube drainage to prevent pleural fibrosis and lung entrapment.
Bronchopleural Fistula
A bronchopleural fistula is a communication between the lung and the pleural space that often originates from a peripheral airway. A bronchopleural fistula from a bronchus typically results in an empyema, whereas an air leak from peripheral airspaces may cause an intractable pneumothorax without associated infection. Bronchopleural fistulas often develop from dehiscence of a bronchial stump following lobectomy or pneumonectomy, or as the result of a necrotizing pulmonary infection. Presenting symptoms include fever, cough, and dyspnea; large air leaks may be noted in patients with pleural drains. Radiographically, a bronchopleural fistula presents as a loculated intrapleural air and fluid collection. An air–fluid level in the postpneumonectomy space should suggest the diagnosis. CT is useful in evaluating patients with suspected bronchopleural fistula and empyema (Fig. 19.8) (6). It can distinguish a hydropneumothorax from a peripheral lung abscess and occasionally demonstrates the actual fistulous communication.
Following pneumonectomy, the residual space gradually fills with fluid and appears radiographically as an opaque hemithorax with ipsilateral mediastinal shift. The radiographic findings suggesting bronchopleural fistula formation complicating pneumonectomy are described in the previous section. CT and MR are useful in evaluating the postpneumonectomy space for evidence of tumor recurrence, and may help in the diagnosis of postoperative bronchopleural fistula and empyema.
Pneumothorax
Pneumothorax results from air entering the pleural space and may be traumatic or spontaneous (Table 19.5). Spontaneous pneumothorax is further subdivided into a primary form, which has no identifiable etiology, and a secondary form, which is associated with underlying parenchymal lung disease (7). Patients with a pneumothorax typically present with the sudden onset of dyspnea and pleuritic chest pain.
Radiographically, pneumothorax on upright radiography is recognized by nondependent lucency that parallels the chest wall and displaces the visceral pleural line medially. In a supine patient, such as in the ER or ICU setting, a pneumothorax can be undetectable as air in the pleural space rises nondependently and creates indiscernible increased lucency over the lower thorax and upper abdomen. Signs of pneumothorax on supine radiography include a hyperlucent upper abdomen (particularly on the right over the normally dense liver), the “deep sulcus” sign (Fig. 19.9), the “double diaphragm” sign, the epicardial fat pad sign (for left pneumothorax), and an unusually sharp heart border. In patients with preexisting pleural adhesions, a pneumothorax can present as a loculated lucency within the pleural space including the interlobar fissures.
On CT, pneumothorax is identified by nondependent lucency over the lower anterior thorax. It is not uncommon in trauma patients to detect a small basilar pneumothorax overlying the lower chest that is not evident radiographically.
Traumatic Pneumothorax. Trauma is the most common cause of pneumothorax. Penetrating injuries can produce pneumothorax by introducing air from the atmosphere into the pleural space or by laceration of the visceral pleura, resulting in an air leak from the lung. Gunshot and knife wounds to the chest and upper abdomen, central line placement, thoracentesis, transbronchial biopsy, and percutaneous needle biopsy are common penetrating injuries that cause traumatic pneumothorax. Blunt chest trauma may cause pneumothorax by two different mechanisms: (1) An acute increase in intrathoracic pressure results in extra-alveolar interstitial air because of alveolar disruption, which tracks peripherally and ruptures into the pleural space. (2) Laceration of the tracheobronchial tree can produce a pneumothorax with a large bronchopleural fistula. In patients with rib fractures, the free edge of the fractured ribs can project inward to lacerate the lung and cause pneumothorax.
Table 19.5 Etiology of Pneumothorax | ||||
|---|---|---|---|---|
|
Primary spontaneous pneumothorax most often occurs in young or middle-aged men. A familial incidence and a propensity for tall, thin individuals has been noted. Affected patients may have blebs or bullae in the lung apices that are responsible for the development of recurrent pneumothoraces. Treatment of the initial episode is with closed tube drainage, with thoracoscopic bullectomy reserved for recurrent episodes or persistent air leak.
Secondary Spontaneous Pneumothorax. Multiple entities have been associated with secondary spontaneous pneumothorax, although in some patients the lungs are intrinsically normal. In the majority of the latter, there is usually a history of sudden increases in intrathoracic pressure. Chronic obstructive pulmonary disease is the most common predisposing condition. Acute obstruction to expiration from bronchoconstriction (asthma) or the performance of the Valsalva maneuver (crack cocaine or marijuana smoking, transvaginal childbirth) may cause spontaneous pneumothorax. Pneumothorax may complicate cystic lung changes in sarcoidosis, Langerhans cell histiocytosis of lung, and lymphangioleiomyomatosis. Necrotizing pneumonia or lung abscess caused by gram-negative or anaerobic bacteria, TB, or Pneumocystis jiroveci pneumonia can lead to pneumothorax, particularly in the mechanically ventilated patient. Metastases to the lung are an infrequent cause of pneumothorax and rarely are a presenting feature of disease. In these cases, pneumothorax develops when necrotic subpleural metastases rupture into the pleural space (Fig. 19.10).
Sarcomas, particularly osteogenic sarcoma, lymphoma, and germ cell malignancies, are the most common primary malignancies to produce spontaneous pneumothorax. Marfan syndrome is the most common connective tissue disease producing pneumothorax; it usually results from the rupture of
apical bullae. Other connective tissue diseases that can produce pneumothorax are Ehlers-Danlos syndrome and cutis laxa.
apical bullae. Other connective tissue diseases that can produce pneumothorax are Ehlers-Danlos syndrome and cutis laxa.
Mechanically ventilated patients are particularly at risk for pneumothorax because of the administration of positive pressure, emphysema, underlying or complicating necrotizing pneumonia, and frequent line placements and other invasive procedures. Not uncommonly, patients with ARDS develop small peripheral cystic airspaces, which can rupture into the pleural space. When these are seen to develop on serial chest radiographs, impending pneumothorax can be suggested.
A particularly rare type of recurrent pneumothorax that occurs with menstruation is catamenial pneumothorax. This condition affects women in their fourth decade and is most likely caused by the cyclical necrosis of pleural endometrial implants, which creates an air leak between the lung and pleura. Rarely, air entering the peritoneal cavity during menstruation gains access to the pleural cavity via diaphragmatic defects. The predilection for right-sided pneumothoraces in this disorder indicates a key role for right-sided diaphragmatic defects. The pneumothoraces tend to be small and resolve spontaneously. Catamenial pneumothorax is managed by preventing menstruation with the administration of oral contraceptives.
Tension pneumothorax is a critical condition that most often results from iatrogenic trauma in mechanically ventilated patients. Tension pneumothorax results from a check-valve pleural defect that allows air to enter but not exit the pleural space. This leads to a pleural air collection that has a pressure exceeding atmospheric pressure during at least a portion of the respiratory cycle, causing complete collapse of the underlying lung and impairing venous return to the heart. Clinically, patients present with tachypnea, tachycardia, cyanosis, and hypotension. Radiographically, the involved hemithorax is expanded and hyperlucent, with a medially retracted lung, ipsilateral diaphragmatic depression or inversion, and contralateral mediastinal shift (Fig. 19.11). It is important to remember that contralateral mediastinal shift from pneumothorax does not invariably indicate tension, since a relative inequality in the degree of negative intrapleural pressure can produce shift in the absence of tension. Therefore, tension pneumothorax remains a clinical diagnosis. Immediate evacuation of the pleural space should be performed with a needle, catheter, or large-bore thoracostomy tube.
Focal Pleural Disease
Focal pleural disease may be divided into localized pleural thickening, pleural calcification, or pleural mass (Table 19.6) (8).
Localized pleural thickening from fibrosis is usually the end result of peripheral parenchymal and pleural inflammatory disease, with pneumonia the most common cause. Additional causes include pulmonary embolism with infarction, asbestos exposure, trauma, prior chemical pleurodesis, and drug-related pleural disease.
Table 19.6 Focal Pleural Disease | ||||||||
|---|---|---|---|---|---|---|---|---|
|
Pleural calcification is most often unilateral and involves the visceral pleura. It is usually the result of prior hemothorax or empyema (e.g., TB), although pleural thickening from any cause may calcify. Asbestos exposure can cause bilateral multifocal calcified parietal pleural plaques. Visceral pleural calcifications from pleural hemorrhage or infection are indistinguishable radiographically. Initially, the calcification is punctate, but it often progresses to become sheetlike. CT is particularly useful in characterizing pleural calcification (Fig. 19.12). The presence of fluid within calcified pleural layers seen on CT suggests an active empyema and is most often seen in patients with prior TB. The use of CT and HRCT in the evaluation of asbestos-related focal pleural disease and calcification is discussed in a subsequent section.
Pleural Mass. Focal pleural masses are usually benign neoplasms such as lipomas; loculated pleural fluid can mimic a pleural mass radiographically. Thoracic lipomas may arise in the chest wall or subpleural fat. Subpleural lipomas produce a pleural mass and can change shape during respiration or with changes in patient positioning because of their pliable nature. Homogeneous fat attenuation on CT scan (-30 to -100 H) is diagnostic (Fig. 19.13).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree