The pleura may be affected by primary neoplasms or become involved by secondary spread of intrathoracic or extrathoracic tumors. Primary pleural tumors account for approximately 10% of all pleural neoplasms, the most common of which include malignant pleural mesothelioma (MPM) and solitary fibrous tumor. Other primary neoplasms include lymphoma, lipomatous tumors such as lipoma and liposarcoma, synovial sarcoma, and vascular sarcomas such as epithelioid hemangioendothelioma. Secondary neoplasms account for approximately 90% of pleural tumors and include metastatic disease and lymphoma. In this chapter we discuss the etiology, clinical presentation, and imaging of these neoplasms, with specific attention given to their appearance on computed tomography (CT), magnetic resonance imaging (MRI), and fluorodeoxyglucose (FDG)–positron emission tomography (PET)-CT.
Malignant Pleural Mesothelioma
Etiology, Prevalence, and Epidemiology
Malignant mesothelioma (MPM) is an uncommon, but increasingly recognized, neoplasm derived from mesothelial cells of the pleura, pericardium, peritoneum, or tunica vaginalis of the testis ; rare cases apparently derived from mediastinal mesothelial cysts have been reported. Mesothelioma is the most common primary malignant neoplasm of the pleura, and it has importance not only because of its poor prognosis but also because of its impact on litigation and worker’s compensation related to occupational asbestos exposure.
In North America and Europe mesothelioma is estimated to account for 20 deaths per million men per year. In Europe the cancer incidence is expected to peak between 2010 and 2030, despite regulatory restrictions adopted during the 1980s and 1990s, and there are 2000 to 3000 new cases per year reported in the United States. Epidemiologic data suggest that the incidence of mesothelioma has already peaked in the United States. In Israel the incidence increased sevenfold from 1.17 per million per year from 1978 to 1980 to 8.5 per million per year from 1993 to 1996. Mesothelioma incidence rates have increased progressively in New Zealand since the 1960s and reached 25 per million for men in 1995. In Japan analyses predict that there will be about 100,000 deaths caused by pleural mesothelioma in the next 40 years. Despite the ban on asbestos use in Western countries, asbestos is still being used in many developing countries. Mesothelioma occurs in both men and women, but overall the incidence is much higher in men. In Denmark, for example, the incidence rate of mesothelioma was 1.33 per 100,000 person-years from 1983 to 1987 among men and 0.51 from 1973 to 1977 among women. The sex predominance is largely related to the increased likelihood of occupational asbestos exposure in men. The industries with the highest risks of mesothelioma are construction and ship repair, asbestos industry, and manufacture of metal construction materials; the occupations at highest risk are plumbers, pipe fitters, and sheet-metal workers. The mean age at diagnosis is 63 years. It is estimated that only 2% to 5% of tumors appear in childhood or adolescence.
Asbestos
There is strong evidence that more than 80% of mesotheliomas are related to the carcinogenic effect of asbestos. However, only approximately 10% of those with asbestos exposure develop mesothelioma. Even minimal exposure to asbestos may result in mesothelioma. Exposure history may be difficult to obtain but can be confirmed by detection of asbestos fibers in the lung. For example, exposure may occur by living in the household of an asbestos worker and coming into contact with fibers brought home on his or her body or clothing. Most exposure to asbestos is by occupation, with the exception of environmental exposure in areas of Greece, Turkey, and New Caledonia, where tremolite exists naturally in the soil in substantial quantities and is used by villagers to whitewash their homes. There is a latency period of 35 to 40 years from time of exposure to development of mesothelioma. Of interest, in the environmental exposure to asbestos in Turkey, a longer latency period of 55 years has been observed. A few cases have been reported after shorter latency periods, but this may be related to earlier unobserved exposures to asbestos.
The principal forms of asbestos include chrysotile, crocidolite, amosite, tremolite, actinolite, and anthophyllite. All but chrysotile (serpentine form) are classified as amphiboles, which tend to have a thin, needle-like appearance. Asbestos is classified as a known human carcinogen by US state, federal, and international agencies, as well as by the World Health Organization. The most carcinogenic fiber is crocidolite, also known as blue asbestos, but the other amphiboles are also associated with mesothelioma. The amphiboles are more likely to be associated with mesothelioma than is chrysotile, or white asbestos, which has a two to four times less potent risk of mesothelioma ; however, the risk for development of lung cancer is equipotent for all types of asbestos. Chrysotile accounts for more than 99% of the world production of asbestos and commonly is contaminated with tremolite and other asbestos fibers.
Asbestos was used in many household and building products in the past, as it is a fibrous silicate that is strong and flexible and does not burn. It was used in insulation, cement, textiles, and ceiling and floor tiles. It is one of the most pervasive environmental hazards in the world, present in more than 3000 manufactured products. The first reported association of mesothelioma with asbestos was published in 1960 by Wagner and colleagues. In 1989 the Environmental Protection Agency announced an asbestos ban and phaseout, but in 1990 the ban was overturned by a US Circuit Court of Appeals. Unfortunately, although the industrialized countries show a steady decrease in asbestos use, the poorer developing countries show an increase. Because of this indiscriminate dispersal of asbestos in the human environment in past years, it is common to find hundreds of thousands to millions of fibers in human lungs. In general, those with heavy exposures have greater asbestos lung burdens. For example, lung tissue taken from patients with mesothelioma often contains more than a million fibers per gram of tissue.
Other Risk Factors
Other fibrous materials, such as a fibrous zeolite and erionite, are strongly associated with mesothelioma, most notably in central Turkey. There is also increased risk of mesothelioma with prior irradiation, with chronic inflammation such as tuberculosis, and in association with the simian virus SV40. This virus was inadvertently transmitted to humans in contaminated poliovirus vaccines in the late 1950s and early 1960s. It is estimated that perhaps 10% to 20% of mesotheliomas may be accounted for by the SV40 virus. It is found in 80% of patients with mesothelioma. Hamster studies indicate that mineral fibers and SV40 are cocarcinogens, which may explain why lower amounts of asbestos are sufficient to cause mesothelioma in individuals infected with SV40. There is also a familial occurrence of mesotheliomas in both asbestos- and erionite-exposed individuals, with mortality rates from mesothelioma as high as 50% in some villages. Whereas cigarette smoking and asbestos are clearly synergistic in the etiology of lung cancer, there is no evidence of a similar role for tobacco in the etiology of mesothelioma.
Clinical Presentation
The onset of MPM is often insidious, and the most common first symptoms are dyspnea and chest pain. Chest pain is often vague and may be referred to the shoulder but typically is nonpleuritic. A substantial number of patients, up to one-third in one series, may present with unilateral pleural effusion. Patients may be asymptomatic, with the presence of the pleural effusion noted incidentally on physical examination or chest radiography. The effusion may be recurrent and present for more than 3 years before the onset of symptoms. An unusual presentation is spontaneous pneumothorax, first described in association with MPM in 1956. In a recent series as many as 10% of MPM cases (9 of 92) presented with a spontaneous pneumothorax. As the disease progresses, patients may develop dry cough, fever, fatigue, and weight loss. Physical examination reveals finger clubbing; retraction and dullness on percussion of the chest are common. The patient may adapt to functioning with only one lung and remain fairly free of symptoms for months or even years, but eventually the tumor usually results in severe pain by invading the chest wall, intercostal nerves, mediastinum, spine, or abdomen. Late manifestations include ascites and chest wall deformity. Median survival time from symptom onset is approximately 1 year, depending on initial stage and various prognostic factors. Local invasion of the chest wall and surrounding structures can cause increasing pain that is difficult to palliate, as well as functional abnormalities, including dysphagia, superior vena cava syndrome, Horner syndrome, vocal cord paralysis, and diaphragmatic paralysis. Death is rarely a result of metastatic disease; it is usually caused by infection or respiratory failure along with constitutional symptoms associated with progressive malignant disease.
Pathology
The mesothelial cell is pluripotent because of its origin from the mesoderm, which is formed from both ectodermal and endodermal layers. Various irritants can cause metaplastic changes of the epithelial type, even squamous metaplasia and connective tissue types. There are three separate histologic subtypes of MPM: the epithelial (epithelioid), sarcomatous (sarcomatoid), and mixed (biphasic) type. The epithelioid type is most common, accounting for approximately 50% to 60% of cases; the sarcomatoid, 35%; and the biphasic form, 15%. On cytologic examination the cells of most epithelioid MPMs are relatively monotonous. On occasion, cells have a foamy, clear, or signet-ring appearance. Sarcomatoid forms resemble sarcomas, most commonly malignant fibrous histiocytoma, although forms resembling osteosarcoma and chondrosarcoma have been reported. Biphasic MPMs can show any combination of the patterns seen in either form.
At the time of surgery and on gross pathologic examination, the parietal pleura is involved to a greater degree than the visceral pleura, as the disease occurs initially on the parietal surface of the pleural mesothelium. The right hemithorax is involved more often than the left. Tumors may be focal or coalesce into sheet-like pleural masses. In the largest postmortem case series of patients with MPM, extrathoracic metastatic disease was present in 55% of cases.
Diagnosis
The histopathologic diagnosis of MPM can be difficult and may require the opinion of an expert pathologist. Common diagnostic dilemmas for the pathologist include differentiation between adenocarcinoma and tubulopapillary MPM, between reactive mesothelial hyperplasia and early MPM, and between desmoplastic MPM and benign pleuritis or pleural plaque. Use of immunohistochemistry stains by an experienced pathologist who has access to sufficient fresh and formalin-fixed tissue will optimize results. In difficult cases electron microscopy remains the gold standard. Epithelioid MPMs possess large numbers of desmosomes and long, slender, branching microvilli, whereas adenocarcinomas have fewer desmosomes and short, stubby, unbranched microvilli. The special stains reported to be most useful include periodic acid–Schiff with diastase, hyaluronic acid, mucicarmine, carcinogenic embryonic antigen, Leu-M1, and more recently calretinin and cytokeratin 5/6.
The diagnosis of MPM can be established by means of thoracentesis with cytology, closed pleural biopsy, and open pleural biopsy by video-assisted thoracic surgery. However, the result of cytology is positive in only 30% to 35% of cases. This diagnostic yield may be improved with the help of immunohistochemistry and electron microscopy. Closed pleural needle biopsy also has a low yield, largely because the biopsy is done in a blinded fashion and may easily miss the tumor. It increases the yield of cytology by only 7% to 26%. Video-assisted thoracic surgery or thoracoscopy has become the favored diagnostic procedure for suspected MPM; it has sensitivity of 91% to 98% but is a costly and invasive technique. The procedure requires 24- to 36-hour admission to the hospital and has a low rate of major complications. An important aspect of MPM is its propensity to spread along tracks of chest tubes, thoracoscopy trocars, and surgical incisions. These implantation metastases are extremely painful and difficult to treat. Seeding along the track after video-assisted thoracoscopic surgery may occur in up to 50% of patients, whereas the highest reported incidence after image-guided biopsy is only 22%. Image-guided needle biopsy has therefore been evaluated as a viable alternative to surgical diagnosis, with a high diagnostic accuracy. It is essential that enough tissue be obtained to permit a reliable diagnosis; fine-needle aspiration biopsy technique is inadequate, but core-needle biopsy is diagnostic in up to 86% of cases. Image-guided biopsy can be performed with ultrasonography, fluoroscopy, or CT; the operator must adequately visualize the tumor and obtain an optimal amount of tissue. In the presence of an effusion, larger samples with use of 16- to 20-gauge cutting needles may be obtained, as the risk of pneumothorax is low. Immunostaining allows a pathologic diagnosis of MPM to be made from a relatively small tissue sample, such as that provided by image-guided core needle biopsy.
Imaging
General Imaging Features
In general, the most common imaging manifestations of MPM include pleural effusion, pleural thickening with or without nodules/masses, ipsilateral volume loss, intrathoracic and extrathoracic lymphadenopathy, and metastatic disease. Findings related to asbestos-related pleural disease may also be seen. Although individual imaging findings may be nonspecific for MPM, the presence of one or more of these features should raise suspicion for MPM, especially in the appropriate clinical scenario.
Radiography
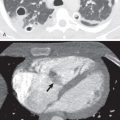
Because of its widespread use and availability, the first modality to depict imaging abnormalities of MPM is often chest radiography. The appearance of MPM on chest radiography ranges from normal in early disease to complete opacification of a hemithorax. A unilateral pleural effusion is one of the most common manifestations and is present in 30% to 80% of patients ( Fig. 74.1 ). Diffuse pleural thickening and pleural nodules/masses are seen in 60% and 45% to 60% of cases, respectively ( Fig. 74.2 ). Extension of tumor along the interlobar fissures may be identified. Encasement of the lung may produce volume loss, resulting in elevation of the ipsilateral hemidiaphragm, ipsilateral mediastinal shift, and narrowing of the intercostal spaces. Osseous or cartilaginous differentiation may produce regions of ossification or calcification in the tumor. An indistinct or “shaggy” cardiac silhouette or ill-defined diaphragmatic contours may be seen in the setting of asbestos-related pleural disease.


Intrathoracic lymphadenopathy may manifest as abnormal thickening of lines and stripes, and the normal mediastinal contours may be absent. Extrathoracic lymphadenopathy and intrathoracic and extrathoracic metastatic disease are better assessed with cross-sectional imaging.
Computed Tomography
CT allows differentiation between benign and malignant pleural disease in most cases. Leung and colleagues studied 74 consecutive patients with diffuse pleural disease. Features that were helpful in distinguishing malignant from benign pleural disease were circumferential pleural thickening, nodular pleural thickening, parietal pleural thickening of more than 1 cm, and mediastinal pleural involvement, with specificities of 100%, 94%, 94%, and 88% and sensitivities of 41%, 51%, 36%, and 56%, respectively. Of 39 malignant cases, 28 were identified correctly by the presence of one or more of these criteria (sensitivity, 72%; specificity, 83%). Metintas and colleagues reviewed the CT findings of 215 patients, 99 with MPM, 39 with metastatic pleural disease, and 77 with benign pleural disease. Sensitivities for circumferential thickening and mediastinal pleural involvement were 70% and 85%, respectively; pleural thickness of more than 1 cm was 47% sensitive and 64% specific. Mediastinal pleural involvement had a lower specificity of 67%, probably explained by the large number of patients with tuberculous pleural infection (42% of benign pleural disease), which may also involve the mediastinal pleura.
CT is the imaging modality of choice to evaluate MPM as it reliably demonstrates the extent of tumor, lymphadenopathy, and intrathoracic and extrathoracic spread and is often sufficient for disease staging and treatment planning. With the administration of intravenous contrast material, malignant or inflammatory pleural disease enhances strongly, enabling differentiation between thickened pleura and effusions as well as atelectasis. Unenhanced CT also allows differentiation of thickened pleura from fluid, whereby the attenuation of the pleura is increased relative to that of the fluid. This may be accentuated by changing the window setting to a narrower window width, such as the liver window. With modern CT scanners, the use of narrow collimation (1 mm or less) allows high-quality multiplanar reconstructions and can be helpful in visualizing involvement of the interlobar fissures or extension below the diaphragm. A unilateral pleural effusion is seen in up to 74% of patients ( Fig. 74.3 ). Pleural thickening can be nodular or lobular in configuration and is identified in 92% of cases ( Fig. 74.4 ). Pleural thickening that is nodular, circumferential, and greater than 1 cm in thickness is highly suggestive of malignant pleural disease, including MPM. In cases of osseous or cartilaginous differentiation, ossification or calcification may be seen in regions of pleural abnormality, and the extent may be scattered or diffuse. MPM with osseous or cartilaginous differentiation appears as large or punctate foci of mineralization within the tumor. Calcified pleural plaques are seen in 10% to 52% of cases and should not be mistaken for MPM with osseous or cartilaginous differentiation. Calcification associated with pleural plaques is linear and can be identified on thickened plaques. There may be other features of asbestos exposure, such as rounded atelectasis and asbestosis in the lungs.


MPM may invade the mediastinum and result in the loss of fat and tissue planes between mediastinal structures. Invasion should also be suspected when MPM encases greater than 50% of the circumference of the trachea, esophagus, or other mediastinal structures ( Fig. 74.5 ). Involvement of the pericardium, which may be nontransmural or transmural, may produce pericardial effusion, pericardial thickening, pericardial nodules/masses, and/or infiltrating soft tissue ( Fig. 74.6 ). Differentiating between nontransmural and transmural involvement may be difficult, although identification of epicardial fat suggests nontransmural involvement. A tumor that extends to the internal surface of the pericardium or involves the myocardium is consistent with transmural disease. Chest wall involvement is commonly seen at the site of previous intervention, including biopsy, drain, and surgical sites. MPM that invades the chest wall may manifest as loss of normal extrapleural fat planes, invasion of intercostal muscles, rib displacement, or osseous destruction ( Fig. 74.7 ). The accuracy of CT for transdiaphragmatic extension remains poor; however, the presence of a distinct fat plane between the inferior surface of the diaphragm and the adjacent abdominal organs is the most reliable indication that MPM is limited to the chest ( Fig. 74.8 ). MPM with multifocal or diffuse invasion of the chest wall, invasion of the mediastinal structures, transmural pericardial disease, involvement of the contralateral pleura, transdiaphragmatic extension, or metastatic disease is typically considered unresectable.




CT remains one of the primary methods for identifying lymph node metastases. Mediastinal lymph nodes, such as paratracheal, hilar, subcarinal, paraesophageal, and paraaortic lymph nodes, that measure 10 mm or larger in short-axis dimension are usually considered abnormal. Internal mammary, retrocrural, and extrapleural lymph nodes have no specific size criteria, and identification of these lymph nodes is often considered abnormal. In MPM different patterns of intrathoracic lymphadenopathy may be encountered, depending on the presence, location, and extent of pleural and diaphragmatic involvement. The anterior pleura is drained by the internal mammary and peridiaphragmatic lymph nodes, the posterior pleura is drained by extrapleural lymph nodes, the anterior and lateral diaphragm is drained by the internal mammary and anterior peridiaphragmatic nodes, and the posterior diaphragm is drained by the paraaortic and posterior mediastinal nodes. The anterior and lateral chest wall lymphatics drain to the anterior or pectoral nodes, and the posterior chest wall lymphatics drain to the axillary nodes ( Fig. 74.9 ).

Intrathoracic and extrathoracic metastatic disease may be demonstrated on CT. Pulmonary metastases appear as nodules or masses, and lymphangitic carcinomatosis manifests as thickening and nodularity of the interlobular septa.
Magnetic Resonance Imaging
A number of studies have assessed the role of MRI in distinguishing malignant from benign pleural disease. By use of the CT criteria for malignant pleural thickening described previously, as well as signal intensity characteristics, sensitivities and specificities of MRI were equivalent to those of CT. High–signal intensity on proton density/T2-weighted MRI in comparison with intercostal muscles provides a valuable additional feature to differentiate benign from malignant pleural disease (100% sensitivity, 87% specificity; negative predictive value, 100%).
A recent study evaluated the ability of texture and shape features on CT and MRI to differentiate benign and malignant pleural lesions and assessed if radiomic models could improve the confidence and accuracy of radiologists with different subspecialty backgrounds. This group found that quantitative textural and shape analysis may help distinguish malignant from benign lesions and concluded that a radiomics-based approach may increase diagnostic confidence of abdominal radiologists and potentially improve radiologists’ accuracy in the assessment of pleural lesions characterized by MRI.
MRI is not routinely used to evaluate patients with MPM but may provide more precise staging information in certain scenarios. Pleural effusions related to MPM are typically hyperintense on T2-weighted images. Pleural thickening is isointense to mildly hyperintense relative to muscle on T1-weighted images and moderately hyperintense relative to muscle on T2-weighted and proton density–weighted images ( Fig. 74.10 ). After the administration of intravenous gadolinium-based contrast material, enhancement is typical (see Fig. 74.10 ). MRI is more sensitive than CT and other imaging modalities in detecting invasion of the chest wall, mediastinum, and diaphragm ( Fig. 74.11 ). Loss of the normal fat planes, extension into mediastinal fat, and tumoral encasement of more than 50% of the circumference of a mediastinal structure are some of the MRI features that suggest tumor invasion.


More recently, it has been shown that diffusion-weighted imaging (DWI) and dynamic contrast-enhanced MRI can further help characterize the histologic cell types of MPM, with the epithelial subtype showing higher apparent diffusion coefficient (ADC) value than the sarcomatoid subtype. It has been proposed that an ADC value of 1.31 × 10 −3 mm 2 /s can be used as a cutoff to differentiate the two histologic cell types. Giesel and colleagues were able to correlate perfusion parameters in mesothelioma with angiogenesis in these tumors.
Fluorodeoxyglucose–Positron Emission Tomography–Computed Tomography
FDG–PET-CT is an important adjunctive examination that is often used for the diagnosis and staging of MPM, as it combines valuable metabolic information and anatomic detail. FDG uptake in MPM and other causes of malignant pleural disease is significantly higher than in benign pleural diseases, and greater FDG uptake has been associated with a shorter survival time ( Fig. 74.12 ). The extent of FDG uptake is associated with median time to tumor progression and survival. Although PET-CT is suboptimal compared with CT and MRI in delineating the extent of local tumor, it may depict invasion of the mediastinum and chest wall. PET-CT is better than CT and MRI for demonstrating intrathoracic and extrathoracic lymphadenopathy (see Fig. 74.12 ) and most cases of metastatic disease. In addition, PET-CT is more accurate than CT, MRI, and PET alone for the staging of MPM.

PET-CT can be used to plan image-guided and surgical biopsies as the locations of greatest FDG uptake and/or those that are most accessible can be identified and targeted for tissue sampling. Another role for PET is in helping to predict prognosis. Higher FDG uptake is associated with significantly shorter survival time. Another study showed good correlation between FDG uptake and surgical stage. This information may be useful clinically in determining the most appropriate treatment. PET-CT is also beneficial for evaluating treatment response ( Figs. 74.13 and 74.14 ) and detecting recurrent disease ( Fig. 74.15 ).



Ultrasonography
Ultrasonography can be useful in identifying disease of the pleura. The pleural effusion acts as an acoustic window and can enable detection of intrapleural nodules. Ultrasonography-guided biopsy of pleural thickening (reported accuracy of 80% for diagnosis of MPM) and drainage of effusions are well-established, safe techniques.
Staging
The eighth edition of the tumor-node-metastasis (TNM) staging system for MPM is effective as of January 1, 2018, by the American Joint Committee on Cancer (AJCC)/Union for International Cancer Control (UICC). Recommendations for the eighth edition are the result of an international collaboration and analysis of databases under the auspices of the International Association for the Study of Lung Cancer (IASLC) ( Tables 74.1 to 74.4 ).
| T Classification | Definition |
|---|---|
| TX | Primary tumor cannot be assessed |
| T0 | No evidence of primary tumor |
| T1 | Tumor limited to the ipsilateral parietal ± visceral ± mediastinal ± diaphragmatic pleura |
| T2 | Tumor involving each of the ipsilateral pleural surfaces (parietal, mediastinal, diaphragmatic, and visceral pleura) with at least one of the following features: (1) involvement of diaphragmatic muscle or (2) extension of tumor from visceral pleura into the underlying lung parenchyma |
| T3 | Describes locally advanced but potentially resectable tumor involving all of the ipsilateral pleural surfaces (parietal, mediastinal, diaphragmatic, and visceral pleura) with at least one of the following features: (1) involvement of the endothoracic fascia; (2) extension into the mediastinal fat; (3) solitary, completely resectable focus of tumor extending into the soft tissues of the chest wall; (4) nontransmural involvement of the pericardium |
| T4 | Describes locally advanced technically unresectable tumor involving all of the ipsilateral pleural surfaces (parietal, mediastinal, diaphragmatic, and visceral pleura) with at least one of the following features: (1) diffuse extension or multifocal masses of tumor in the chest wall, with or without associated rib destruction; (2) direct transdiaphragmatic extension of tumor to the peritoneum; (3) direct extension of tumor to the contralateral pleura; (4) direct extension of tumor to mediastinal organs; (5) direct extension of tumor into the spine; (6) tumor extending through to the internal surface of the pericardium with or without a pericardial effusion; or (7) tumor involving the myocardium |
| Regional Lymph Nodes (N) | Definition |
|---|---|
| NX | Regional lymph nodes cannot be assessed |
| N0 | No regional lymph node metastases |
| N1 | Metastases in the ipsilateral bronchopulmonary, hilar, or mediastinal lymph nodes (including the internal mammary, peridiaphragmatic, mediastinal fat pad, or intercostal lymph nodes) |
| N2 | Metastases in the contralateral bronchopulmonary, hilar, or mediastinal lymph nodes or ipsilateral or contralateral supraclavicular lymph nodes |
| Metastasis (M) | Definition |
|---|---|
| M0 | No distant metastasis |
| M1 | Distant metastasis present |
| N0 | N1 | N2 | |
|---|---|---|---|
| T1 | IA | II | IIIB |
| T2 | IB | II | IIIB |
| T3 | IB | IIIA | IIIB |
| T4 | IIIB | IIIB | IIIB |
| M1 | IV | IV | IV |
T Classification
The extent of primary tumor spread, which includes tumor size, invasion, and location, is of primary importance in determining prognosis. The T component of staging for MPM is based entirely upon the location of tumor tissue and anatomic site of invasion. In contrast to the staging of other malignancies, measurements are not used. However, unidimensional measurements of pleural thickness suggest that quantitative measures of disease burden may have prognostic value, and further work is planned to assess whether this limited preliminary data may add important information to the staging system in the future.
Regarding specific T descriptors, T1 describes tumor affecting the ipsilateral parietal plus/minus visceral plus/minus mediastinal plus/minus diaphragmatic pleura. T2 lesions involve all ipsilateral pleural surfaces and at least one additional feature, such as invasion of the diaphragm or extension into the lung parenchyma. T3 disease includes all ipsilateral pleural surfaces and at least one additional feature, such as involvement of the endothoracic fascia, invasion of the mediastinal fat, a solitary, resectable focus of chest wall invasion, or involvement of the pericardium without transmural invasion. Finally, T4 tumors involve all pleural surfaces and at least one additional feature, such as multifocal chest wall invasion, with or without rib destruction; direct transdiaphragmatic extension into the peritoneum; direct extension to the contralateral pleura; direct invasion of mediastinal organs; direct invasion of the spine; and transmural involvement of the pericardium, with or without pericardial effusion.
N Classification
The N component of the MPM staging system was originally based on the N staging for lung cancer; however, it should be noted that lymphatic drainage of the pleura is distinct from that of the lung. The eighth edition is the first evidence-based system for staging of lymph nodes in MPM. Lymph node metastases occur in 35% to 50% of MPM patients who undergo surgical resection; an autopsy series found lymph node involvement in 76%. Lymph node metastases can decrease long-term survival by up to 50%.
N1 disease includes metastases involving ipsilateral bronchopulmonary, hilar, mediastinal, internal mammary, peridiaphragmatic/pericardial, and intercostal lymph nodes. N2 comprises metastases involving contralateral bronchopulmonary, hilar, and mediastinal lymph nodes, as well as metastases involving any supraclavicular lymph node. Metastatic disease to the axillary lymph nodes is considered M1 disease and is not included in the N descriptor. The staging of lymph nodes is difficult, given the limited accuracy of the size criteria used on CT, and metastases may be present in lymph nodes measuring less than 1 cm. Mediastinoscopy has been shown to be more accurate than CT in the staging of MPM, with a sensitivity of 80% (vs. 60% for CT), a specificity of 100% (vs. 71% for CT), and an accuracy of 93% (vs. 67% for CT), although some lymph node groups are not accessible with this method. Cervical mediastinoscopy allows evaluation of the 2R (upper right paratracheal), 2L (upper left paratracheal), 4R (lower right paratracheal), 4L (lower left paratracheal), and 7 (subcarinal) stations. Extended mediastinoscopy permits evaluation of the 5 (subaortic) and 6 (paraaortic) stations.
M Classification
The M component of the staging system is binary—distant metastases are present (M1) or absent (M0). Metastatic disease is a late manifestation of disease, and some of the most common sites of metastatic spread include the contralateral pleura, contralateral lung, peritoneum/abdomen, extrathoracic lymph nodes (apart from supraclavicular lymph nodes), bone, liver, and brain. Initial results of the IASLC Mesothelioma Staging Project suggest that the number of metastatic lesions may have an effect on prognosis, and further research on this topic may inform future editions of the TNM classification.
Prognosis
The prognosis overall is extremely poor. Most patients die within the first year of the onset of symptoms, and “long-term survival” is rare. In two series of 167 patients and 114 patients, only 7 (2.5%) lived for more than 5 years. Two-year and 5-year survival rates are reported as 23% and 0%, respectively, in another study of 80 patients. As for most other cancers, prognosis is related to the extent of local or distant tumor spread. Prognosis is better in tumors with an epithelioid subtype than with sarcomatoid histology and appears to be independent of stage. Many factors are also reported to be prognostic in MPM, including histologic features, age, gender, and performance status, as well as chest pain, dyspnea, platelet count greater than 400,000/µL, weight loss, serum lactate dehydrogenase level greater than 500 U/L, low hemoglobin level, high white blood cell count, and increasing age older than 75 years all carry a worse prognosis.
Treatment
Different treatment strategies may be used for MPM, depending on tumor factors, such as histology and stage at diagnosis, and patient factors, such as age and performance status. Single-modality therapy (i.e., surgery, chemotherapy, or radiation therapy alone) has demonstrated limited survival benefit. Therefore current treatment regimens focus on multimodality therapy, which has shown greater survival benefit.
Chemotherapy
Most patients with MPM are diagnosed at advanced stages of disease. Thus chemotherapy is the mainstay of therapy, and a regimen of platinum-based cisplatin and the antifolate pemetrexed is considered first-line therapy for unresectable disease. This combination results in longer median survival and longer time to disease progression than treatment with cisplatin alone. Other treatment regimens include gemcitabine and vinorelbine, alone or in combination with cisplatin. Neoadjuvant cisplatin may be used with pemetrexed or gemcitabine before extrapleural pneumonectomy and adjuvant radiation therapy.
Surgery
Surgical treatments are typically used early on (stages I and II) and include pleurectomy and decortication and extrapleural pneumonectomy. Pleurectomy and decortication involve removal of the parietal and visceral pleura from the lung surface, mediastinum, pericardium, diaphragm, and chest wall. Extrapleural pneumonectomy is characterized by en bloc resection of the lung, parietal and visceral pleura, ipsilateral hemidiaphragm, and ipsilateral pericardium. A recent study showed no statistically significant differences in survival for either procedure at any stage of disease. However, multivariate analysis demonstrated significant survival benefit for pleurectomy and decortication versus extrapleural pneumonectomy. Pleurectomy and decortication are associated with a higher postsurgical recurrence rate than extrapleural pneumonectomy, although metastatic disease is less common.
Radiation Therapy
Radiation therapy is typically used for palliative purposes in MPM. One study showed decreased tumor seeding of procedural tracts with use of radiation therapy; however, prophylactic radiation therapy for this purpose is not currently recommended. Adding intensity-modulated radiation therapy to extrapleural pneumonectomy may result in a decrease in local-regional recurrence. Radiation treatment plans include all preoperative pleural surfaces, the ipsilateral mediastinal lymph nodes, the retrocrural space, and the deep margin of the surgical incision.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree