Clinical Presentation
The patient is a 70-year-old woman with metastatic breast cancer who presented with paresthesias with a cold sensation and burning dysesthesias in both feet. Decreased sensation in both legs extends from her feet to her groin. Paresthesias also involve the left forearm. She has difficulty buttoning clothing. The patient has had two recurrences of metastatic adenopathy in the supraclavicular, mediastinal, and perihilar regions bilaterally for which she received two courses of chemotherapy and radiation therapy. These treatments were completed approximately 1 year ago.
Imaging Presentation
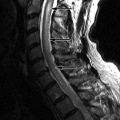
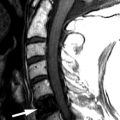
Sagittal T1-weighted image reveals homogeneous increased signal intensity throughout the vertebral marrow and a mildly enlarged cervical spinal cord. Sagittal T2-weighted image shows a long segment of abnormal increased T2 signal intensity within the cervical spinal cord from C3-C7. These findings are consistent with radiation-induced spinal cord edema causing myelopathy ( Fig. 59-1 ) .

Discussion
Radiation therapy is a mainstay in the treatment of neoplasms of the spine. However, the use of radiation must be balanced with its potential side effects. Radiation can cause disturbance in bone growth in the immature skeleton. Complications in the mature skeleton include osteoradionecrosis, radiation-induced fractures, and radiation-induced neoplasms. Radiation can also have an effect on the intraspinal and paraspinal soft tissues. Radiation myelopathy/myelitis is a very serious complication.
Histologically, there are two phases of bone marrow changes from radiation. In the acute phase, radiation causes edema, vascular congestion, and capillary injury. These changes can be seen within 1 to 3 days of the initiation of radiation therapy. In the chronic phase, hematopoietic cells and blood vessels are depleted and replaced by yellow fat cells. These changes can be seen within 2 to 6 weeks of the initiation of radiation therapy. Radiation changes in bone depend on the patient’s age, absorbed dose, size of the radiation field, beam energy, and fractionation.
Radiation to the immature skeleton can cause asymmetric vertebral growth and fibrosis of the overlying soft tissues. This asymmetry can lead to scoliosis. Radiation-induced scoliosis is convex to the side opposite the radiation port and occurs in up to 80% of skeletally immature patients. The degree of scoliosis is related to the dose of radiation and is more severe in patients who have received radiation under 2 years of age.
In skeletally mature patients, radiation impairs osteoblast function resulting in decreased matrix production. Radiographically, this is seen as osteopenia, which is typically seen 1 year after radiation and can lead to bone atrophy. Attempts at repair of the damaged bone result in deposition of new bone on ischemic trabecula. Fractures may occur through this weakened bone. The constellation of radiographic findings of osteopenia, areas of increased density from attempts at repair, and coarsened trabecula has been called radiation osteitis , radiation necrosis , or osteoradionecrosis ). These findings can appear similar to radiation-induced sarcoma; however, osteoradionecrosis can be differentiated from sarcoma by the absence of soft tissue mass seen with osteoradionecrosis, changes confined to the radiation field, and its stability over time.
Radiation can give rise to benign and malignant neoplasms. Osteochondromas are the most common benign radiation-induced tumor and are histologically and radiographically identical to spontaneously occurring osteochondromas. Radiation-induced osteochondromas are most commonly found in children who were irradiated at less than 2 years of age, with a latent period from 17 months to 9 years. Radiation can rarely give rise to malignant sarcomas, the majority of which are osteosarcomas followed by fibrosarcomas. They can arise in both preexisting bone lesions and in bones that were normal prior to radiation therapy. The latency period is from 11 to 14 years. The diagnosis of radiation-induced sarcoma is established by four criteria: (1) a long latency period, (2) malignancy with the radiation field, (3) pathologic evaluation demonstrating sarcomatous change, and (4) the radiation-induced sarcoma must differ histologically from the original lesion that was treated with radiation. Patients with sarcomas are often symptomatic, presenting with pain and swelling, often with a palpable soft-tissue mass.
Radiation myelopathy causes injury to the white matter of the spinal cord. Patients usually present 9 to 15 months after the end of radiation. Clinically, the patient can present early on with paresthesia (particularly with an inability to perceive pain and temperature). As it progresses, the patient can experience various symptoms including gait abnormalities and hemiplegia. Several factors influence the development of radiation myelopathy including the total delivered dose of radiation, fractionation of the radiation dose (fractionation increases the latent period), the volume of the irradiated tissue (larger volumes decrease the latent period), the linear energy transfer (the greater the transfer the more likely myelopathy is going to occur), and the level of the spinal cord irradiated (posterior and lateral spinal cord involvement in the cervical/upper thoracic region, anterior and lateral spinal cord involvement in the lower thoracic/lumbar region). Three criteria must be satisfied to diagnose radiation myelopathy: (1) the affected spinal cord must have been within the radiation field, (2) the neurologic deficit must correspond to the affected spinal cord segment, and (3) metastases or other primary spinal cord lesions must have been excluded.
Imaging Features
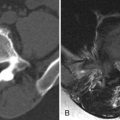
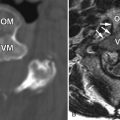
Marrow-related changes from radiation therapy are best seen with magnetic resonance imaging (MRI). Within the first 6 weeks after radiation, the marrow signal reflects changes of bone edema and vascular congestion. Therefore, the marrow may demonstrate slightly decreased to no change on T1-weighted images and hyperintensity on short tau inversion recovery (STIR) or T2-weighted images, particularly if a fat-suppression technique is used. There is also enhancement of the marrow in the early stages. After 6 weeks, the more typical appearance of increased T1 signal secondary to fatty marrow and intermediate signal intensity on T2-weighted images is found ( Fig. 59-1 ). There is no contrast enhancement in the late phase. One also finds that there is a sharply demarcated bandlike appearance on T1-weighted images of the portions of the spine that are within the radiation field ( Fig. 59-2 ) . This same radiation portal can be “seen” as a sharp region of photopenia on bone scan.

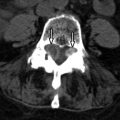
Radiation myelopathy is also best seen with MRI. In the acute phase of radiation myelopathy, the spinal cord is expanded and demonstrates decreased to intermediate T1 signal and increased T2 signal (see Fig. 59-1 ). Contrast enhancement is variable and may be patchy or ringlike ( Fig. 59-3 ) . In the chronic phase of radiation myelopathy, the affected portions of the spinal cord (often the regions that enhanced) can become atrophic.