In adults, the most common expansile “mass” lesion in the posterior fossa is a subacute stroke, whereas the most common neoplastic lesion in the posterior fossa is cerebellar metastasis (intra-axial) or vestibular schwannoma (extra-axial). Those diseases fall outside the scope of this article, which focuses on primary intra-axial tumors of the posterior fossa in adults. This category of tumors is uncommon and more frequently encountered in children. This article reviews tumors of the cerebellum, brainstem, and fourth ventricle that are seen in adult patients, following categories from the 2007 World Health Organization classification of central nervous system tumors.
- •
In the adult posterior fossa, the most common “mass” is a subacute stroke, the most common tumor is a cerebellar metastasis, and the most common primary tumor is hemangioblastoma.
- •
Medulloblastoma and pilocytic astrocytoma can be seen in adults as well as children; low-grade and high-grade diffuse gliomas can be seen in the brainstem as well as in the cerebral hemispheres.
- •
Tumors of the fourth ventricle may originate from the wall (ependymoma, subependymoma, or rosette-forming glioneuronal tumor [RGNT]) or from the lumen (choroid plexus papilloma or carcinoma, meningioma, or metastasis).
- •
In the adult posterior fossa, the most common “mass” is a subacute stroke, the most common tumor is a cerebellar metastasis, and the most common primary tumor is hemangioblastoma.
- •
Medulloblastoma and pilocytic astrocytoma can be seen in adults as well as children; low-grade and high-grade diffuse gliomas can be seen in the brainstem as well as in the cerebral hemispheres.
- •
Tumors of the fourth ventricle may originate from the wall (ependymoma, subependymoma, or rosette-forming glioneuronal tumor [RGNT]) or from the lumen (choroid plexus papilloma or carcinoma, meningioma, or metastasis).
Adult pilocytic astrocytoma is a rare diagnosis, with incidence of less than 0.1 case per 100,000 person-years in adults over age 45 years. Analysis of 3066 pilocytic astrocytomas from the National Cancer Institute Surveillance, Epidemiology, and End Results database found differences in location and prognosis between adult and pediatric patients. Although the cerebellum was the most common site in children (37%), it was slightly outnumbered by the cerebral lobes in adults (27% vs 30%). And although the cancer-specific 5-year survival rates were excellent in children (>95%), they dropped to 92%, 79%, and 64% within the 20 to 39, 40 to 59, and 60+ age groups, respectively. Molecular analysis in a large series of 127 adult pilocytic astrocytomas found the BRAF-KIAA1549 fusion gene in only 20%—whereas BRAF-KIAA1549 fusion is present in greater than 60% of pediatric cases and may confer a less aggressive clinical phenotype. Despite a higher recurrence rate in adults (30%), standard treatment is resection without adjuvant radiation.
FABP4
There have also been a few case reports of supratentorial or extracerebellar liponeurocytomas, which are analogous to infratentorial or cerebellar liponeurocytomas on radiology (except for location), histopathology, and immunohistochemistry. Given the predilection for the lateral ventricles in younger adults, supratentorial liponeurocytomas are not currently part of the 2007 WHO classification and may be considered a rare lipidized variant of central neurocytoma. Other case reports have described familial (an underlying germline mutation has not been identified yet) and multifocal cerebellar liponeurocytomas. Treatment is surgical resection; however, up to 50% of patients have local recurrence on long-term follow-up. Adjuvant radiotherapy to the posterior fossa has been recommended for subtotal resections and for any liponeurocytomas with unusually aggressive histology (Ki-67 proliferative index >6%).
Neuronal tumors: rosette-forming glioneuronal tumor of the fourth ventricle (WHO grade I)
In 2002, Komori and colleagues reported “eleven cases of a distinctive tumor of the posterior fossa [with] relatively discrete, focally enhancing mass(es) primarily involving the aqueduct, fourth ventricle, and cerebellar vermis.” They were mixed neuronal-glial tumors, characterized by biphasic architecture with neurocytic and astrocytic components. Similar lesions had previously been classified as dysembryoplastic neuroepithelial tumors of the cerebellum; however, there were important histopathologic differences, for example, the formation of neurocytic rosettes and perivascular pseudorosettes as well as the frequent presence of a pilocytic astrocytoma-like component. These differences led to a new entity of RGNT of the fourth ventricle, officially introduced in the most recent 2007 WHO classification “as a rare, slowly growing tumor of the fourth ventricular region that predominantly affects young adults (mean age 33).”
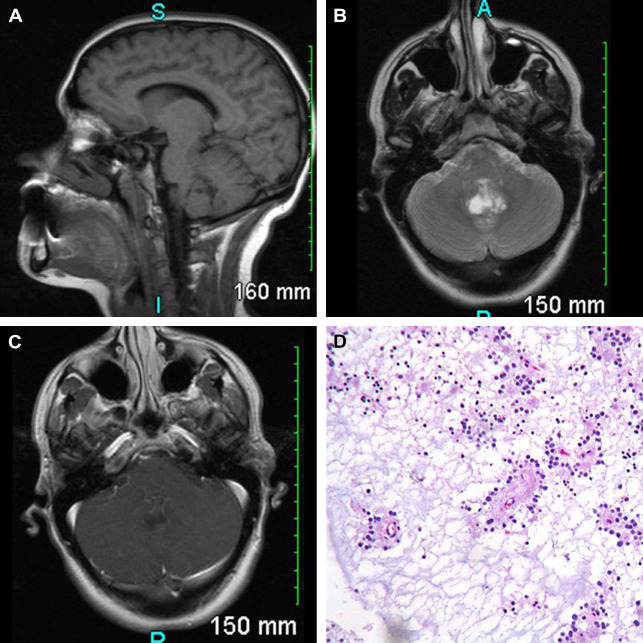
In 1 meta-analysis of 41 patients with RGNT, the most common presenting symptoms were headaches (68%) and ataxia (39%), with frequent obstructive hydrocephalus (44%). Most are midline lesions of the posterior fossa; they are thought to arise from pluripotential cells in the subependymal plate at the ventricular wall ( Fig. 10 ). These tumors can be solid or mixed solid-cystic and often demonstrated focal contrast enhancement (82%). There is usually heterogeneous hypointensity on T1 and hyperintensity on T2 weighted images, with hypointense foci on T2* weighted images (gradient-recalled echo/susceptibility-weighted imaging) related to intratumoral calcification or hemorrhage. Both the occurrence of satellite lesions and drop metastases through CSF dissemination have been described. There have also been case reports of RGNT arising outside the fourth ventricle, most commonly in the pineal region, and around other CSF-containing spaces from third ventricle to central canal.
Disclosures: None.
References
- 1. Roller L.A., Bruce B.B., and Saindane A.M.: Demographic confounders in volumetric MRI analysis: is the posterior fossa really small in the adult Chiari 1 malformation? AJR Am J Roentgenol 2015; 204: pp. 835-841
- 2. Barkovich J., and Raybaud C.: Pediatric neuroimaging. Philadelphia (PA): Lippincott Williams & Wilkins, 2012.
- 3. Louis D.N., Ohgaki H., Wiestler O.D., et al: The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol 2007; 114: pp. 97-109
- 4. Neumann H.P., Eggert H.R., Weigel K., et al: Hemangioblastomas of the central nervous system. A 10-year study with special reference to von Hippel-Lindau syndrome. J Neurosurg 1989; 70: pp. 24-30
- 5. Lonser R., and Oldfield E.: Hemangioblastomas. In Winn R. (eds): Youmans neurological surgery, 6th edition. Philadelphia (PA): Saunders, 2011. pp. 1389-1399
- 6. Courcoutsakis N.A., Prassopoulos P.K., and Patronas N.J.: Aggressive leptomeningeal hemangioblastomatosis of the central nervous system in a patient with von Hippel-Lindau disease. AJNR Am J Neuroradiol 2009; 30: pp. 758-760
- 7. Kim H., Park I.S., and Jo K.W.: Meningeal supratentorial hemangioblastoma in a patient with von hippel-lindau disease mimicking angioblastic menigioma. J Korean Neurosurg Soc 2013; 54: pp. 415-419
- 8. Doyle L.A., and Fletcher C.D.: Peripheral hemangioblastoma: clinicopathologic characterization in a series of 22 cases. Am J Surg Pathol 2014; 38: pp. 119-127
- 9. Park D.M., Zhuang Z., Chen L., et al: von Hippel-Lindau disease-associated hemangioblastomas are derived from embryologic multipotent cells. PLoS Med 2007; 4: pp. e60
- 10. Lee S.R., Sanches J., Mark A.S., et al: Posterior fossa hemangioblastomas: MR imaging. Radiology 1989; 171: pp. 463-468
- 11. Ho V.B., Smirniotopoulos J.G., Murphy F.M., et al: Radiologic-pathologic correlation: hemangioblastoma. AJNR Am J Neuroradiol 1992; 13: pp. 1343-1352
- 12. Lonser R.R., Vortmeyer A.O., Butman J.A., et al: Edema is a precursor to central nervous system peritumoral cyst formation. Ann Neurol 2005; 58: pp. 392-399
- 13. Slater A., Moore N.R., and Huson S.M.: The natural history of cerebellar hemangioblastomas in von Hippel-Lindau disease. AJNR Am J Neuroradiol 2003; 24: pp. 1570-1574
- 14. Hoang M.P., and Amirkhan R.H.: Inhibin alpha distinguishes hemangioblastoma from clear cell renal cell carcinoma. Am J Surg Pathol 2003; 27: pp. 1152-1156
- 15. Shonka N., Brandes A., and De Groot J.F.: Adult medulloblastoma, from spongioblastoma cerebelli to the present day: a review of treatment and the integration of molecular markers. Oncology (Williston Park) 2012; 26: pp. 1083-1091
- 16. Koeller K.K., and Rushing E.J.: From the archives of the AFIP: medulloblastoma: a comprehensive review with radiologic-pathologic correlation. Radiographics 2003; 23: pp. 1613-1637
- 17. Pomeroy S.L., Tamayo P., Gaasenbeek M., et al: Prediction of central nervous system embryonal tumour outcome based on gene expression. Nature 2002; 415: pp. 436-442
- 18. Bourgouin P.M., Tampieri D., Grahovac S.Z., et al: CT and MR imaging findings in adults with cerebellar medulloblastoma: comparison with findings in children. AJR Am J Roentgenol 1992; 159: pp. 609-612
- 19. Koci T.M., Chiang F., Mehringer C.M., et al: Adult cerebellar medulloblastoma: imaging features with emphasis on MR findings. AJNR Am J Neuroradiol 1993; 14: pp. 929-939
- 20. Majós C., Alonso J., Aguilera C., et al: Adult primitive neuroectodermal tumor: proton MR spectroscopic findings with possible application for differential diagnosis. Radiology 2002; 225: pp. 556-566
- 21. Smoll N.R.: Relative survival of childhood and adult medulloblastomas and primitive neuroectodermal tumors (PNETs). Cancer 2012; 118: pp. 1313-1322
- 22. Riffaud L., Saikali S., Leray E., et al: Survival and prognostic factors in a series of adults with medulloblastomas. J Neurosurg 2009; 111: pp. 478-487
- 23. Levy R.A., Blaivas M., Muraszko K., et al: Desmoplastic medulloblastoma: MR findings. AJNR Am J Neuroradiol 1997; 18: pp. 1364-1366
- 24. Remke M., Hielscher T., Northcott P.A., et al: Adult medulloblastoma comprises three major molecular variants. J Clin Oncol 2011; 29: pp. 2717-2723
- 25. Perreault S., Ramaswamy V., Achrol A.S., et al: MRI surrogates for molecular subgroups of medulloblastoma. AJNR Am J Neuroradiol 2014; 35: pp. 1263-1269
- 26. Lee Y.Y., Van Tassel P., Bruner J.M., et al: Juvenile pilocytic astrocytomas: CT and MR characteristics. AJR Am J Roentgenol 1989; 152: pp. 1263-1270
- 27. Koeller K.K., and Rushing E.J.: From the archives of the AFIP: pilocytic astrocytoma: radiologic-pathologic correlation. Radiographics 2004; 24: pp. 1693-1708
- 28. Johnson D.R., Brown P.D., Galanis E., et al: Pilocytic astrocytoma survival in adults: analysis of the surveillance, epidemiology, and end results program of the National Cancer Institute. J Neurooncol 2012; 108: pp. 187-193
- 29. Theeler B.J., Ellezam B., Sadighi Z.S., et al: Adult pilocytic astrocytomas: clinical features and molecular analysis. Neuro Oncol 2014; 16: pp. 841-847
- 30. Hawkins C., Walker E., Mohamed N., et al: BRAF-KIAA1549 fusion predicts better clinical outcome in pediatric low-grade astrocytoma. Clin Cancer Res 2011; 17: pp. 4790-4798
- 31. Ellis J.A., Waziri A., Balmaceda C., et al: Rapid recurrence and malignant transformation of pilocytic astrocytoma in adult patients. J Neurooncol 2009; 95: pp. 377-382
- 32. Stüer C., Vilz B., Majores M., et al: Frequent recurrence and progression in pilocytic astrocytoma in adults. Cancer 2007; 110: pp. 2799-2808
- 33. Fulham M.J., Melisi J.W., Nishimiya J., et al: Neuroimaging of juvenile pilocytic astrocytomas: an enigma. Radiology 1993; 189: pp. 221-225
- 34. de Fatima Vasco Aragao M., Law M., Batista de Almeida D., et al: Comparison of perfusion, diffusion, and MR spectroscopy between low-grade enhancing pilocytic astrocytomas and high-grade astrocytomas. AJNR Am J Neuroradiol 2014; 35: pp. 1495-1502
- 35. Laigle-Donadey F., Doz F., and Delattre J.Y.: Brainstem gliomas in children and adults. Curr Opin Oncol 2008; 20: pp. 662-667
- 36. Reyes-Botero G., Mokhtari K., Martin-Duverneuil N., et al: Adult brainstem gliomas. Oncologist 2012; 17: pp. 388-397
- 37. Landolfi J.C., Thaler H.T., and DeAngelis L.M.: Adult brainstem gliomas. Neurology 1998; 51: pp. 1136-1139
- 38. Purohit B., Kamli A.A., and Kollias S.S.: Imaging of adult brainstem gliomas. Eur J Radiol 2015; 84: pp. 709-720
- 39. Guillamo J.S., Monjour A., Taillandier L., et al: Brainstem gliomas in adults: prognostic factors and classification. Brain 2001; 124: pp. 2528-2539
- 40. Theeler B.J., Ellezam B., Melguizo-Gavilanes I., et al: Adult brainstem gliomas: correlation of clinical and molecular features. J Neurol Sci 2015; 353: pp. 92-97
- 41. Reithmeier T., Kuzeawu A., Hentschel B., et al: Retrospective analysis of 104 histologically proven adult brainstem gliomas: clinical symptoms, therapeutic approaches and prognostic factors. BMC Cancer 2014; 14: pp. 115
- 42. Mabray M.C., Glastonbury C.M., Mamlouk M.D., et al: Direct cranial nerve involvement by Gliomas: case series and review of the literature. AJNR Am J Neuroradiol 2015; 36: pp. 1349-1354
- 43. Bi Z., Ren X., Zhang J., et al: Clinical, radiological, and pathological features in 43 cases of intracranial subependymoma. J Neurosurg 2015; 122: pp. 49-60
- 44. Yachnis A., and Rivera-Zengotita M.: Ependymomas and subependymoma. In Yachnis A., and Rivera-Zengotita M. (eds): Neuropathology. Philadelphia (PA): Saunders, 2014. pp. 99-106
- 45. Smith A.B., Smirniotopoulos J.G., and Horkanyne-Szakaly I.: From the radiologic pathology archives: intraventricular neoplasms: radiologic-pathologic correlation. Radiographics 2013; 33: pp. 21-43
- 46. Hoeffel C., Boukobza M., Polivka M., et al: MR manifestations of subependymomas. AJNR Am J Neuroradiol 1995; 16: pp. 2121-2129
- 47. Frazier J., and Jallo G.: Intracranial ependymomas in adults. In Winn R. (eds): Youmans neurological surgery, 6th edition. Philadelphia (PA): Saunders, 2011. pp. 1383-1388
- 48. Rudà R., Gilbert M., and Soffietti R.: Ependymomas of the adult: molecular biology and treatment. Curr Opin Neurol 2008; 21: pp. 754-761
- 49. Spoto G.P., Press G.A., Hesselink J.R., et al: Intracranial ependymoma and subependymoma: MR manifestations. AJR Am J Roentgenol 1990; 154: pp. 837-845
- 50. Koeller K.K., Sandberg G.D., and Armed Forces Institute of Pathology : From the archives of the AFIP. Cerebral intraventricular neoplasms: radiologic-pathologic correlation. Radiographics 2002; 22: pp. 1473-1505
- 51. Mirzadeh Z., Bina R., Kusne Y., et al: Predictors of functional recovery in adults with posterior fossa ependymomas. J Neurosurg 2014; 120: pp. 1063-1068
- 52. Corbett J., Haines D., Ard M., et al: The ventricles, choroid plexus, and cerebrospinal fluid. Fundamental neuroscience for basic and clinical applications. Philadelphia (PA): Saunders, 2013. pp. 82-94
- 53. Coates T.L., Hinshaw D.B., Peckman N., et al: Pediatric choroid plexus neoplasms: MR, CT, and pathologic correlation. Radiology 1989; 173: pp. 81-88
- 54. Steven D.A., McGinn G.J., and McClarty B.M.: A choroid plexus papilloma arising from an incidental pineal cyst. AJNR Am J Neuroradiol 1996; 17: pp. 939-942
- 55. Milhorat T.H., Hammock M.K., Davis D.A., et al: Choroid plexus papilloma. I. Proof of cerebrospinal fluid overproduction. Childs Brain 1976; 2: pp. 273-289
- 56. Dangouloff-Ros V., Grevent D., Pagès M., et al: Choroid plexus neoplasms: toward a distinction between carcinoma and papilloma using arterial spin-labeling. AJNR Am J Neuroradiol 2015; 36: pp. 1786-1790
- 57. Sharifi G., Bakhtevari M.H., Alghasi M., et al: Bilateral choroid plexus metastasis from papillary thyroid carcinoma: case report and review of the literature. World Neurosurg 2015; 84: pp. 1142-1146
- 58. Koeller K.K., and Henry J.M.: From the archives of the AFIP: superficial gliomas: radiologic-pathologic correlation. Armed Forces Institute of Pathology. Radiographics 2001; 21: pp. 1533-1556
- 59. Meltzer C.C., Smirniotopoulos J.G., and Jones R.V.: The striated cerebellum: an MR imaging sign in Lhermitte-Duclos disease (dysplastic gangliocytoma). Radiology 1995; 194: pp. 699-703
- 60. Smith R.R., Grossman R.I., Goldberg H.I., et al: MR imaging of Lhermitte-Duclos disease: a case report. AJNR Am J Neuroradiol 1989; 10: pp. 187-189
- 61. Cianfoni A., Wintermark M., Piludu F., et al: Morphological and functional MR imaging of Lhermitte-Duclos disease with pathology correlate. J Neuroradiol 2008; 35: pp. 297-300
- 62. Awwad E.E., Levy E., Martin D.S., et al: Atypical MR appearance of Lhermitte-Duclos disease with contrast enhancement. AJNR Am J Neuroradiol 1995; 16: pp. 1719-1720
- 63. Klisch J., Juengling F., Spreer J., et al: Lhermitte-Duclos disease: assessment with MR imaging, positron emission tomography, single-photon emission CT, and MR spectroscopy. AJNR Am J Neuroradiol 2001; 22: pp. 824-830
- 64. Douglas-Akinwande A.C., Payner T.D., and Hattab E.M.: Medulloblastoma mimicking Lhermitte-Duclos disease on MRI and CT. Clin Neurol Neurosurg 2009; 111: pp. 536-539
- 65. Williams D.W., Elster A.D., Ginsberg L.E., et al: Recurrent Lhermitte-Duclos disease: report of two cases and association with Cowden’s disease. AJNR Am J Neuroradiol 1992; 13: pp. 287-290
- 66. Giorgianni A., Pellegrino C., De Benedictis A., et al: Lhermitte-Duclos disease. A case report. Neuroradiol J 2013; 26: pp. 655-660
- 67. Tan T.C., and Ho L.C.: Lhermitte-Duclos disease associated with Cowden syndrome. J Clin Neurosci 2007; 14: pp. 801-805
- 68. Bechtel J.T., Patton J.M., and Takei Y.: Mixed mesenchymal and neuroectodermal tumor of the cerebellum. Acta Neuropathol 1978; 41: pp. 261-263
- 69. Kleihues P., Louis D.N., Scheithauer B.W., et al: The WHO classification of tumors of the nervous system. J Neuropathol Exp Neurol 2002; 61: pp. 215-225
- 70. Jackson T.R., Regine W.F., Wilson D., et al: Cerebellar liponeurocytoma. Case report and review of the literature. J Neurosurg 2001; 95: pp. 700-703
- 71. Nishimoto T., and Kaya B.: Cerebellar liponeurocytoma. Arch Pathol Lab Med 2012; 136: pp. 965-969
- 72. Alkadhi H., Keller M., Brandner S., et al: Neuroimaging of cerebellar liponeurocytoma. Case report. J Neurosurg 2001; 95: pp. 324-331
- 73. Beizig N., Ziadi S., Ladib M., et al: Cerebellar liponeurocytoma: case report. Neurochirurgie 2013; 59: pp. 39-42
- 74. Anghileri E., Eoli M., Paterra R., et al: FABP4 is a candidate marker of cerebellar liponeurocytomas. J Neurooncol 2012; 108: pp. 513-519
- 75. George D.H., and Scheithauer B.W.: Central liponeurocytoma. Am J Surg Pathol 2001; 25: pp. 1551-1555
- 76. Chakraborti S., Mahadevan A., Govindan A., et al: Supratentorial and cerebellar liponeurocytomas: report of four cases with review of literature. J Neurooncol 2011; 103: pp. 121-127
- 77. Wolf A., Alghefari H., Krivosheya D., et al: Cerebellar liponeurocytoma: a rare intracranial tumor with possible familial predisposition. Case report. J Neurosurg 2016; 125: pp. 57-61
- 78. Scoppetta T.L., Brito M.C., Prado J.L., et al: Multifocal cerebellar liponeurocytoma. Neurology 2015; 85: pp. 1912
- 79. Chung S.B., Suh Y.L., and Lee J.I.: Cerebellar liponeurocytoma with an unusually aggressive histopathology: case report and review of the literature. J Korean Neurosurg Soc 2012; 52: pp. 250-253
- 80. Komori T., Scheithauer B.W., and Hirose T.: A rosette-forming glioneuronal tumor of the fourth ventricle: infratentorial form of dysembryoplastic neuroepithelial tumor? Am J Surg Pathol 2002; 26: pp. 582-591
- 81. Zhang J., Babu R., McLendon R.E., et al: A comprehensive analysis of 41 patients with rosette-forming glioneuronal tumors of the fourth ventricle. J Clin Neurosci 2013; 20: pp. 335-341
- 82. Medhi G., Prasad C., Saini J., et al: Imaging features of rosette-forming glioneuronal tumours (RGNTs): a series of seven cases. Eur Radiol 2016; 26: pp. 262-270
- 83. Xu J., Yang Y., Liu Y., et al: Rosette-forming glioneuronal tumor in the pineal gland and the third ventricle: a case with radiological and clinical implications. Quant Imaging Med Surg 2012; 2: pp. 227-231
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree