Abstract
The most common cause of fetal bladder outlet obstruction is posterior urethral valves. Ultrasound examination shows a dilated, thick-walled bladder, often with dilatation of the proximal urethra—the “keyhole” sign. Renal findings may include hydronephrosis, cortical cysts, or kidneys that are small and echogenic. Ureteral dilatation is also common. Oligohydramnios before midgestation confers an extremely poor prognosis and prompts consideration for fetal vesicoamniotic shunt therapy. Important factors before vesicoamniotic shunt placement are that the anomaly appears isolated, that the fetus is male, that the chromosomal microarray (or karyotype) results are normal, and urinary analyte values obtained via serial vesicocentesis suggest relatively preserved renal function. Families need to be counseled about the high rates of acute and chronic morbidity associated with this condition.
Keywords
keyhole sign, cortical cysts, urinary analytes, vesicoamniotic shunt, bladder outlet obstruction
Introduction
Posterior urethral valves (PUV) are the most common cause of fetal lower urinary tract obstruction, also called bladder outlet obstruction. Membranous tissue in the posterior urethra results in varying degrees of obstruction. The condition occurs only in males, although other etiologies of bladder outlet obstruction can affect females and males. There is a wide range of severity prenatally and postnatally. Lack of amniotic fluid during development may result in pulmonary hypoplasia, and many who survive have significant kidney and bladder damage. Severely affected fetuses, including those with oligohydramnios before midgestation, have a grim prognosis in the absence of therapy, with mortality from pulmonary hypoplasia as high as 95%. In selected cases, placement of a fetal vesicoamniotic shunt can restore amniotic fluid volume and avoid lethal pulmonary hypoplasia. However, affected infants still require ablation of the valves, and more than 50% of survivors have renal insufficiency, chronic voiding problems, and other major morbidities. Timely referral is crucial because therapy is not an option if renal function is already severely impaired. PUV have characteristic ultrasound (US) findings that permit prenatal diagnosis, often by the early second trimester and occasionally in the first trimester.
Disorder
Definition
PUV refers to membranous tissue within the male posterior urethra resulting in varying degrees of lower urinary tract obstruction.
Prevalence and Epidemiology
The reported prevalence of PUV is 1 : 8000 to 1 : 25,000 boys. However, this range likely underestimates the number of affected fetuses. In the era before routine prenatal diagnosis, 45% of neonates with PUV died soon after birth from pulmonary hypoplasia. This has been termed the “hidden mortality” of the condition, because affected infants did not survive to reach treatment centers.
Associated anomalies have been reported in more than 40% of fetuses with PUV, including cardiac malformations, gastrointestinal abnormalities, and other genitourinary anomalies. In addition, 5% to 8% of cases of bladder outlet obstruction are associated with aneuploidy.
Etiology and Pathophysiology
The etiology of PUV is postulated to be failure of the urogenital membrane to disintegrate completely, leaving membranous tissue within the posterior urethra. Endoscopic evaluation in affected infants demonstrates a simple mucosal membrane, with scant fibrous stroma, and a small eccentric opening. The major risk is back-pressure damage, which may result in vesicoureteral reflux, cystic renal dysplasia, altered tubular function, and abnormal bladder function.
Manifestations of Disease
Clinical Presentation
In the absence of US evaluation, the fundal height may lag behind gestational age owing to decreased amniotic fluid volume. The prenatal diagnosis is based on US findings.
Imaging Technique and Findings
Ultrasound.
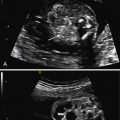
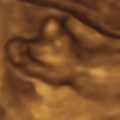
The initial US finding is typically a dilated, thick-walled bladder, which may be evident in the first trimester or early second trimester ( Fig. 14.1 ). The bladder wall is considered abnormal if it measures more than 2 mm thick. Color Doppler of the umbilical arteries as they encircle the bladder may assist with accurate measurement of bladder wall thickness ( Fig. 14.2 ). A characteristic finding is the “keyhole” sign, which refers to the appearance of the dilated proximal urethra contiguous with the bladder (see Fig. 14.2 and Fig. 14.3 ).



The amniotic fluid volume is often severely decreased. Fetal urine production is the major source of amniotic fluid after 16 to 18 weeks’ gestation. If severity of disease is uncertain—which is common—it may be prudent to follow the pregnancy closely around this gestational age range because the amniotic fluid volume may precipitously decrease. Although fetuses with normal amniotic fluid are not at risk for pulmonary hypoplasia, or for the deformations of the face and extremities that characterize Potter sequence, affected infants remain at risk for renal insufficiency, and voiding difficulties.
PUV is characterized by a spectrum of renal findings that reflect both the severity and the timing of the obstructive process. Early and severe obstruction may result in kidneys that are small and echogenic ( Fig. 14.4 ). Kidneys may also have cortical cysts or appear diffusely cystic secondary to obstructive cystic dysplasia, a function of back-pressure damage from obstruction ( Figs. 14.5 and 14.6 ). Renal echogenicity and cystic dysplasia are almost always found in the setting of severe oligohydramnios. In a metaanalysis of infants with bladder outlet obstruction, renal echogenicity, and cortical cysts were the US parameters most predictive of poor renal function, with a sensitivity of 57% and specificity of 84%. Oligohydramnios was also associated with a poor prognosis. Absence of these findings, however, does not guarantee a normal outcome.



Hydronephrosis is a common finding and is often accompanied by ureteral dilatation (see Fig. 14.6 ). It has limited accuracy in predicting outcome. The degree of hydronephrosis may be asymmetric—one side may appear significantly more affected than the other ( Fig. 14.7 ). Occasionally, pressure may cause a rupture along the urinary tract, resulting in a perinephric urinoma or, more commonly, urinary ascites ( Fig. 14.8 ).