A spectrum of cardiopulmonary diseases can be treated surgically. Surgical procedures in the thorax include lung resection, lung transplantation, cardiovascular and mediastinal surgery, as well as pleural and cavitary space reduction procedures. This chapter reviews the surgical procedures, indications, and imaging findings in the postoperative setting, both expected and unexpected, including potential complications. In the intensive care unit, postoperative findings, such as malposition of support lines and tubes, and cardiopulmonary diseases, such as pulmonary edema, pneumonia, pneumothorax, pleural and pericardial fluid collections, and pulmonary embolism, are described in other chapters.
Thoracotomy and Chest Wall Surgery
Description
Thoracotomy is defined as a surgical incision into the chest. In posterolateral thoracotomy a large transverse incision along the lateral aspect of the chest via the fifth intercostal space transects the latissimus dorsi. This incision starts at the anterior axillary line, continues posteriorly beneath the tip of the scapula, and then extends caudally to terminate between the spine and the medial border of the scapula. Typically, in patients older than 40 years, the ribs at the incision site are resected at the costovertebral angle to prevent rib fractures. A thoracoabdominal incision involves a posterolateral incision via the seventh or eighth intercostal space with extension of the incision anteriorly through the costal arch and into the abdomen. In axillary thoracotomy or mini-thoracotomy, a linear incision from the anterior axillary line inferomedially to the midhemithorax avoids the division of several muscles, most importantly the latissimus dorsi. In anterior or anterolateral thoracotomy the incision starts at the midaxillary line and follows the inframammary fold to terminate in the parasternal location. A median sternotomy involves a midline incision from the suprasternal notch to the xiphoid process. In limited anterior thoracotomy the incision length is restricted generally to less than 10 cm. “Clamshell” incision combines bilateral anterolateral thoracotomies with transverse sternotomy. Video-assisted thoracoscopic surgery (VATS) consists of the use of a fiberoptic camera and surgical instruments placed via three to five intercostal incisions along the anterior and posterior axillary lines. More recently, uniportal VATS using a single incision for access rather than multiple incisions has been developed without incurring additional perioperative complications. Advances in robotic technology have led to the amalgamation of robotic surgical techniques with the VATS procedure, now known as robot-assisted thoracic surgery (RATS).
Chest wall surgery aims to resect the lesion with margins wide enough to eradicate the disease, while at the same time leaving a defect that can be closed effectively. The two goals of chest wall reconstruction are to cover the defect and to restore chest wall rigidity. Tissue defect coverage is most commonly accomplished with the use of flaps. A soft tissue flap may contain bone, fascia, muscle, fat, or a combination of those. A flap can be transferred from a donor site to a recipient site (free flap), with surgical development of a new vascular supply, or it can be rotated into position, with its vascular supply maintained through a pedicle connected to the donor site. Free flaps require delicate microsurgical technique to ensure adequate perfusion. Pedicle flaps are more commonly used in thoracic tumor resections because the surgical procedure is less complex and postoperative perfusion is more reliable. Flap nomenclature includes the type of tissue transferred, the distance to be transferred (local or distant), and the numbers and types of pedicles. Myocutaneous and omental flaps are most commonly used in thoracic surgery. Omental flaps are usually brought to the thorax in the retrosternal location via a surgical defect in the anterior diaphragm. To restore chest wall rigidity, prosthetic material (meshes, metals) or autogenous tissue, such as fascia lata, may be used. Important characteristics of effective synthetic material include rigidity, malleability, inertness (to allow ingrowth of fibrous tissue), and radiolucency (to allow easier imaging reassessment).
Indications, Contraindications, Purpose, and Underlying Mechanisms
Median sternotomy allows wide access for anterior mediastinal surgery and is the incision of choice for open-heart surgery. A posterolateral thoracotomy is the standard approach for lung resection and surgery involving the esophagus, posterior mediastinum, and aorta. The thoracoabdominal incision is used in esophageal surgery. An axillary thoracotomy is commonly used for pulmonary lobar resection; an anterior thoracotomy is commonly used for open-lung biopsies and in some esophageal surgeries. A limited anterior thoracotomy is used in minimally invasive cardiac surgery. Clamshell incision is primarily used in bilateral lung transplantation. In the past clamshell thoracotomy was also used to allow access to both lungs in the resection of bilateral primary or metastatic malignancies, which is now frequently achieved by VATS. VATS has become increasingly popular for both diagnostic and therapeutic procedures.
Chest wall resection is most commonly indicated for chest wall tumors, either primary or metastatic. Between 5% and 8% of lung cancers involve the parietal pleura and chest wall, requiring complete resection of the affected chest wall. Other common indications for chest wall resection include radiation necrosis, severe infections, and trauma. To fix chest wall defects at the resection site, chest wall reconstruction is indicated for all full-thickness skeletal defects that have the potential of paradoxical chest wall motion. Chest wall reconstruction is also performed to correct chest wall deformities, most often congenital, such as pectus excavatum and cleft sternum, where failed midline fusion and resultant separation of the sternum leaves the heart and great vessels unprotected.
Indications for intrathoracic flaps include repair of tracheoesophageal fistulas; buttressing of tracheal, bronchial, and esophageal anastomoses; repair of esophageal perforations; repair of bronchopleural fistulas; filling of tissue defects; reinforcement of the chest wall; and space obliteration after treatment of postpneumonectomy empyema. Omental flaps deliver a reliable blood supply and are commonly used to fill the residual space left from muscle flaps and for treatment of poststernotomy mediastinitis and chronic deep tissue infections.
Expected Appearance on Relevant Modalities
On initial postoperative imaging, skin staples are noted in the operative site. Titanium staples used in current automated staplers minimize artifacts on computed tomography (CT) and magnetic resonance imaging. In the first few weeks after thoracotomy, CT can identify minimal soft tissue swelling and subcutaneous air in the operative site. In the long term, CT may also show atrophy of denervated muscles at the incision site. Imaging after chest wall resection and reconstruction can demonstrate mild to marked musculoskeletal distortion, depending on the type and extent of surgery, and large reconstructions can cause mediastinal shift.
After median sternotomy, four to seven stainless steel sternal wires are visible in a vertical configuration. Imaging of the sternum can also show gaps, step-offs (ventral-dorsal misalignment), and impaction (overriding of sternal halves). Although little or no sign of healing of the sternum is expected on imaging in the first few postoperative months, by 1 year, healing is visualized as a continuum of cortical bone over the sternotomy site. Minor sternal irregularities can persist. Within the first week after sternotomy, air can be visible on radiography as subcutaneous linear lucencies in the chest and neck or as small focal air pockets in the mediastinum or retrosternal space, which is best visualized on lateral radiography. Retrosternal air can persist for weeks. Retrosternal soft tissue thickening and mediastinal widening resulting from small amounts of fluid in the mediastinum or pericardium are also commonly seen within the first postoperative week. These expected radiographic changes in the early postoperative period after sternotomy may confound identification of mediastinitis.
Intrathoracic flaps often produce changes in the contour of the heart and mediastinum on radiography. The omental flap frequently appears as a soft tissue opacity in the retrosternal or perihilar region. On CT the omental graft typically has linear opacities representing omental vessels and increased attenuation of the fat caused by edema, resulting in a “smoky” appearance. The omental flap can be followed from the abdominal cavity through the anterior diaphragm to its final intrathoracic location. The thymic and mediastinal fat pad flaps typically appear as fat-attenuation tissue adjacent to the proximal airways on CT, although thymic tissue also may have a component of soft tissue attenuation, depending on the age of the patient.
Intrathoracic muscle flaps have a soft tissue component and, when used in the oncologic setting, can be confused with tumor recurrence if their characteristic features are not recognized. Muscle flaps can have various appearances on imaging because of the vascular pedicles and muscle configurations in characteristic locations. Anterior serratus and latissimus dorsi flaps are usually identified in the posterolateral chest and can mimic empyema. A rectus abdominis muscle flap is generally seen in the inferior retrosternal region. Unilateral pectoral flaps produce a difference in opacity between the hemithoraces on chest radiography. Intercostal muscle flaps are most often identified in the paraspinal region or azygoesophageal recess and typically arise from the posterolateral chest wall. On CT intercostal flaps have a curvilinear shape with a component of fat attenuation and linear calcification, presumably from remnants of periosteum. Flaps can atrophy because of muscle denervation and disuse and show fatty infiltration over time on CT. Gore-Tex, methyl methacrylate, and stainless steel mesh are often used in flap surgery for chest wall defects and are identified on CT as high-attenuation linear opacities of variable thickness beneath muscle flaps.
Potential Complications and Radiologic Appearance
Complications of chest wall surgery include flail chest, winged scapula, fistula formation, incisional dehiscence, wound infection, seroma, and flap failure, as well as lung and bowel herniation.
Dehiscence, the premature opening of any surgical site, is due to poor wound healing ( Fig. 68.1 ). Dehiscence can result from or precipitate wound infection. Risk factors include diabetes, advanced age, obesity, and trauma during the postoperative period. A sizable dehiscence can be identified radiographically as a gap between approximated tissues that may progressively widen over time. A midsternal stripe (midline vertical radiolucency) that widens over time should also raise suspicion. Reorientation of sternal wires on chest radiography is noted in up to 90% of patients with dehiscence. Sternal wire displacement is the single best radiologic sign of dehiscence, allowing early detection. However, a repeat sternotomy may also lead to reorientation of sternal wires. Fractured and displaced wires may migrate into the parasternal soft tissues or, rarely, elsewhere in the thorax.

Wound infection after chest wall surgery and thoracotomy includes both soft tissue and bone infections. Wound infection occurs in almost 5% of chest wall reconstructions as prosthetic mesh is an excellent substrate for bacterial growth. Soft tissue infection and osteomyelitis after sternotomy are important clinical problems. CT findings of sternal infection include parasternal soft tissue stranding, sinus tracts, sternal erosions, and abscess formation. CT sinography can be performed to assess sinus tract depth and to show any mediastinal communication. Early sternal osteomyelitis is difficult to differentiate from expected minor sternal irregularities after surgery and may not become fully apparent until it has reached a late stage.
Seroma is a localized serous fluid collection in the operative site. It is a common problem in the immediate postoperative period and is identified after procedures involving mesh and Silastic implants. On CT seroma appears as a fluid attenuation ovoid collection. Seroma is visualized on ultrasonography as an anechoic fluid collection. Seroma typically resolves after repeated aspiration or spontaneously within a few weeks but may persist and develop a serosal lining that prevents dead space closure and proper wound healing.
Flap failure can be partial or complete. Partial flap failure involves loss of function in only a part of the flap. Flap hemorrhage and infection can lead to partial flap failure and can appear radiographically as an increase in flap size. Total flap failure occurs almost exclusively after complete vascular pedicle disruption, leading to flap necrosis. Lung herniation through the intercostal space is a rare complication caused by lack of chest wall integrity. Lateral and oblique radiographs may show lung parenchyma protruding outside the osseous thorax. CT is more sensitive and clearly shows the location and extent of the hernia ( Fig. 68.2 ). Lung herniation is typically benign but may rarely cause lung strangulation. Bowel herniation may occur after any surgery with an omental flap because the procedure creates a potential passageway for bowel loops through the anterior diaphragm. Herniation is usually a straightforward diagnosis manifested radiographically by loops of bowel extending into the thorax.

Pulmonary Resection
Description
Pulmonary resection involves removal of lung parenchyma, ligation of the vessels supplying the targeted lung section, and resection of the associated bronchi at the most proximal location through thoracotomy, VATS, or RATS. In some cases pulmonary resection procedures may also include resection of parts of the chest wall, diaphragm, pericardium, and associated vasculature.
Wedge resection or nonanatomic sublobar resection consists of the removal of a small wedge-shaped piece of lung tissue, usually through VATS. Segmentectomy is the anatomic resection of the lung based on the segmental vessels and airways and can be performed through a limited thoracotomy or VATS. Lobectomy involves the resection of an entire lobe of the lung, its surrounding visceral pleura, and associated airways and pulmonary vasculature. It can be performed via open thoracotomy, VATS, or RATS. A variant of the lobectomy involves a sleeve resection of the bronchus. The proximal and distal edges of the remaining bronchus are reattached with an end-to-end anastomosis.
Pneumonectomy, performed through a posterolateral thoracotomy, VATS, or RATS, consists of resection of an entire lung along with its visceral pleura and includes hilar dissection and ligation of the ipsilateral main bronchus, pulmonary artery, and superior and inferior pulmonary veins. Pneumonectomy can be intrapericardial or extrapericardial, depending on whether the hilum is dissected inside or outside the pericardial sac. The mainstem bronchial stump after pneumonectomy is typically covered with adjacent tissue (pleura, omentum) to decrease the likelihood of an air leak. In contrast, extrapleural pneumonectomy consists of the resection of the parietal pleura, ipsilateral diaphragm, and pericardium, in addition to the lung and visceral pleura.
Lung volume reduction surgery involves the resection of emphysematous upper lobe lung tissue, most often by wedge resection. Lung volume reduction surgery can be performed through a median sternotomy or VATS and is typically performed bilaterally. Bullectomy and blebectomy consist of the removal of lung bullae or blebs, respectively. A giant bullectomy refers to the resection of a single dominant lung bulla; an apical bullectomy involves the resection of lung bullae located in the lung apices. The major difference between a bleb and bullae is that a bleb is a collection of air within layers of visceral pleura rather than within the lung parenchyma. Bullectomy and blebectomy are usually accomplished through wedge resection as part of VATS.
Indications, Contraindications, Purpose, and Underlying Mechanisms
Wedge resection is used for indeterminate pulmonary nodules, pulmonary metastases, and stage I lung cancers in selected patients unable to tolerate larger resection, including older patients and those with reduced cardiopulmonary function. Wedge resection is also used, instead of an anatomic resection, in those with additional subsolid lesions concerning for adenocarcinomas and those with prior lung resection, to help preserve lung parenchyma and pulmonary function. Wedge resections, usually of more than one lobe, are also used to obtain tissue for pathologic diagnosis in individuals with diffuse lung disease. Segmentectomy is primarily indicated for patients with suppurative pulmonary lesions, such as tuberculosis and bronchiectasis, but it is also used in selected lung cancer patients. Sleeve resection is used to treat tumors involving the junction of lobar bronchi in the right upper lobe, left upper lobe, and left lower lobe. Lobectomy is the surgical resection of choice for early stage non–small cell lung cancer. Pneumonectomy is indicated for cancer that is centrally located, adherent to hilar structures, or crosses a fissure. Pneumonectomy is occasionally performed for extensive inflammatory disease. Completion pneumonectomy can be performed when lung cancer recurs within a previously surgically treated lung. In contrast, extrapleural pneumonectomy is primarily reserved for pleural mesothelioma. Lung volume reduction surgery is used as palliative surgery for selected patients with severe debilitating pulmonary emphysema despite optimal medical management. A giant bullectomy is indicated if a bulla occupies more than 30% of a lung, causes dyspnea, and overlies normal lung parenchyma. Apical bullectomy and blebectomy can be performed to treat pneumothorax resulting from bulla or bleb rupture that causes clinical symptoms.
Expected Appearance on Relevant Modalities
After pulmonary resection, imaging shows characteristic staple lines at the operative site. Focal atelectasis, edema, and hemorrhage at the resection margin manifest as an area of increased opacity or density. This density typically decreases and resolves within days to weeks. Over time, granulation tissue manifesting as soft tissue can occur at the resection margin. The pleural space initially fills with fluid and over time closes by a number of compensatory mechanisms. These mechanisms include expansion of remaining lung, narrowing of intercostal spaces, elevation of the diaphragm, mediastinal shift, and inward displacement of the chest wall. The degree to which these changes occur is related to the extent of pulmonary resection and to the patient’s underlying cardiovascular disease.
After wedge resection and segmentectomy, there is typically complete obliteration of the pleural space within several days by the expansion of the ipsilateral lung. After lobectomy, the pleural space is obliterated within 1 week, primarily through ipsilateral lung expansion and rotation, although minor diaphragmatic elevation and mediastinal shift also contribute. At times, a small amount of fluid in the pleural space may persist and radiographically mimic lobar collapse in the lobectomy site. The remaining lung will show compensatory hyperinflation with increased radiolucency and more widely spaced vascular markings. A small postlobectomy pneumothorax or hydrothorax is frequently visible on chest radiography in the immediate postoperative period. The juxtaphrenic peak sign, a small triangular opacity projecting superiorly over the medial half of the diaphragm or near its highest point is a finding that occurs in about half of patients after upper lobectomy. Its prevalence increases gradually during the weeks after lobectomy, and it is more common on the right.
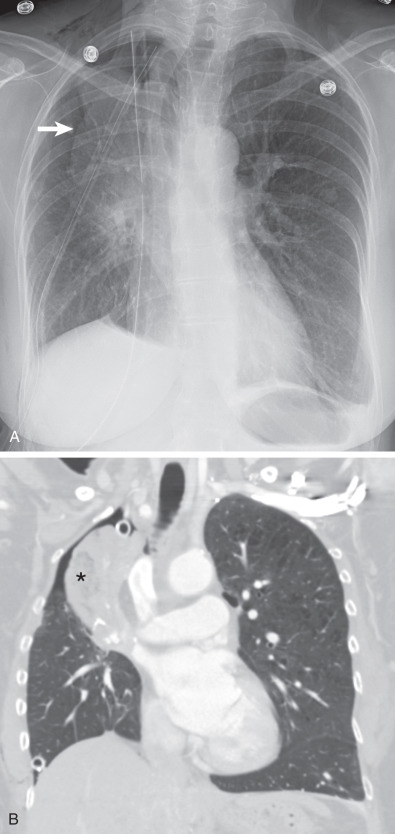
Initially after surgery, the postpneumonectomy space contains mostly air and a small amount of fluid. During the first postoperative week, between one-half and two-thirds of the hemithorax gradually fills with fluid, and air is slowly resorbed ( Fig. 68.3 ). Complete opacification of the hemithorax through compensatory methods typically occurs within 3 to 7 months but may occur in weeks. In contrast, after extrapleural pneumonectomy the surgical space may fill faster, as quickly as 1 week, because of the absence of fluid resorption after excision of the pleura. The air-fluid level slowly rises, and although a small volume of air may persist at the apex without clinical significance; the postpneumonectomy space is typically obliterated within weeks to months. Over time, up to one-third of postpneumonectomy patients have complete pleural fluid resorption, leaving mediastinal structures and thickened fibrous tissue. In the remaining two-thirds, imaging demonstrates a variably sized fluid collection. The trachea and mediastinal structures remain in the midline in the early postoperative period and then gradually shift during the course of weeks to months toward the side of surgery. The degree of shift depends primarily on the compliance of the contralateral lung. Those with a residual fluid collection generally have less mediastinal shift. Stump thrombosis is an in situ thrombus encountered incidentally on late postoperative surveillance CT in the residual pulmonary artery stump after pneumonectomy ( Fig. 68.4 ). It occurs in approximately 10% of postpneumonectomy cases and is more common in the right pulmonary artery stump. Stump thrombosis can also uncommonly occur after lobectomy. Management of this condition with anticoagulation remains controversial.
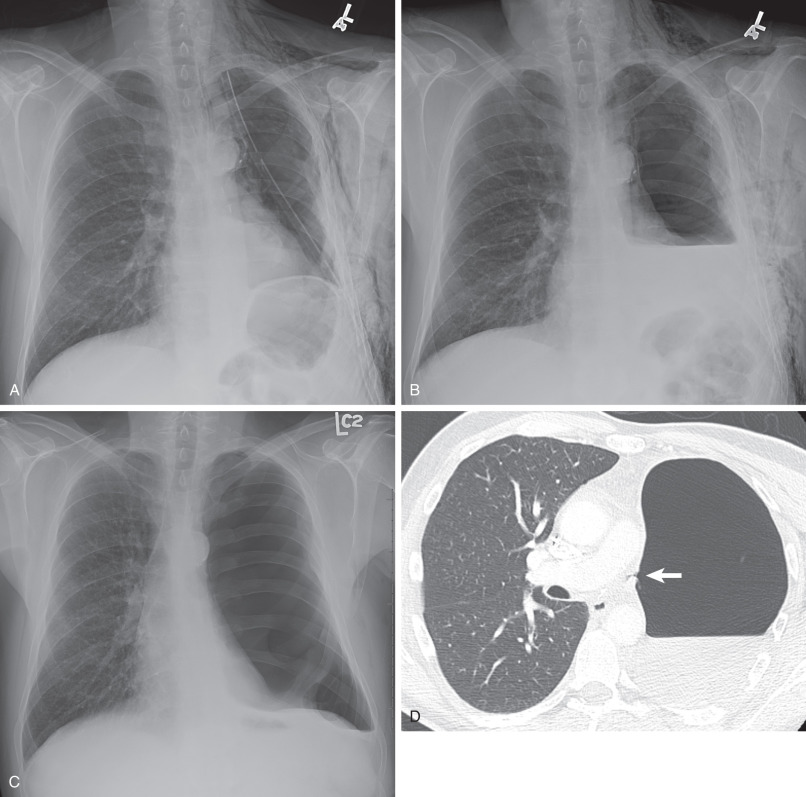

After lung volume reduction surgery, the chest radiograph shows a higher position and greater curvature of the diaphragm and a decrease in diameter of the middle to lower anteroposterior rib cage. The diameter of the transverse rib cage shows little or no decrease in size. CT studies have demonstrated that the intrathoracic trachea typically decreases in length and increases in axial area during the course of several months after lung volume reduction surgery.
Potential Complications and Radiologic Appearance
Air leaks are an important clinical complication after pulmonary resection, particularly when highly friable emphysematous lung has undergone surgical manipulation ( Fig. 68.5 ). After lung volume reduction surgery, invagination of visceral pleura can result in triangular fluid and air collections in the pleural space and lead to atypical manifestations of pneumothorax, pleural effusions, and pulmonary abnormalities. In particular, subpulmonic pneumothorax is a unique and frequent occurrence after lung volume reduction surgery.

After pulmonary resection, an unexpected contralateral or absence of expected ipsilateral mediastinal shift after surgery may indicate an underlying pathologic process. For example, absence of mediastinal shift to the left after left pneumonectomy may indicate an expansion of the pneumonectomy space by air, blood, chyle, or fluid. Underlying bronchopleural fistula, empyema, and/or tumor growth should be considered ( Fig. 68.6 ). An additional sign of volume expansion is a change from the normal convex curve of the mediastinum to a concave curve on the affected side. This sign is most commonly seen with empyema. Alternatively, volume loss in the contralateral lung, such as pulmonary fibrosis, which prevents compensatory hyperinflation and atelectasis, can sometimes account for lack of expected shift of the mediastinum to the side of lung resection. CT is the modality of choice to identify the pathologic process underlying abnormal mediastinal shifts.

Bronchopleural fistula after pulmonary resection is a communication between the bronchial stump and the pleural space, resulting from dehiscence at the bronchial stump. This complication typically occurs within the first 2 weeks after surgery, although it may occur months later. The reported incidence of bronchopleural fistula that follows pulmonary resection varies but averages around 5% to 8%, 3% to 6%, and 0.5% to 1% after extrapleural pneumonectomy, pneumonectomy, and lobectomy, respectively. Risk factors for bronchopleural fistula include inflammatory disease, preoperative radiation therapy, residual malignancy at the bronchial stump, diabetes, and resection involving the right lung.
Bronchopleural fistula (BPF) leads to pneumothorax, and a late appearance predisposes patients to infection of the pleural space, resulting in empyema ( Fig. 68.7 ). On chest radiography a decrease in the air-fluid level greater than 2 cm in the postpneumonectomy space is a sensitive indicator of BPF. Other indicators of BPF include the development of a new air-fluid level and the expansion of a loculated pleural air collection in the operative site. CT with thin-section images on the order of 1 to 2 mm can sometimes demonstrate the fistulous connection from the bronchial stump to the pleural space (see Fig. 68.3 ). Indirect signs such as small bubbles of air around the bronchial stump may also suggest BPF.

Empyema can be identified on radiography in the early postoperative period by rapid fluid filling of the pneumonectomy space and contralateral shift of the mediastinum. CT shows expansion of the pneumonectomy space with fluid, which can be complex, with intermediate attenuation and with mass effect on the adjacent heart and mediastinum. CT also frequently demonstrates irregular thickening of the parietal pleura with enhancement after administration of intravenous contrast ( Fig. 68.8 ).

Postpneumonectomy syndrome is an uncommon complication with marked ipsilateral mediastinal shift resulting in compression of the contralateral mainstem bronchus that typically occurs in children and young adults months to years after pneumonectomy ( Fig. 68.9 ). The syndrome is almost exclusively seen after right pneumonectomy. CT of postpneumonectomy syndrome usually demonstrates a marked rightward and posterior displacement of the mediastinum with counterclockwise rotation of the heart and great vessels. CT also shows left lung hyperinflation with significant displacement of the left lung into the anterior right hemithorax. As the trachea and left mainstem bronchus are rotated to the right, they are stretched and compressed by both the aortic arch and left pulmonary artery anteriorly and by the descending aorta and vertebral column posteriorly. Symptoms include stridor caused by airway narrowing and recurrent infection caused by impaired clearance of secretions. This can be treated by placing tissue expanders or breast implants into the pneumonectomy space to reposition the mediastinum toward midline.

Lobar torsion is a rare and serious complication after lobectomy in the early postoperative period that arises from rotation of a remaining lobe on its bronchovascular pedicle. The resultant compression of pulmonary vasculature can lead to pulmonary infarction and death. The earliest radiographic finding is lobar atelectasis that is due to bronchial compression in the twisted lobe. Vascular compression quickly leads to lobar congestion, which is visualized on chest radiography as a rapidly expanding opacity in the affected lobe. On CT the torsed lobe demonstrates increased volume and consolidation and decreased enhancement because of poor arterial flow. Additional CT findings include tapering and obliteration of the proximal pulmonary artery and associated bronchus and a component of ground-glass opacity in the affected lobe. Right middle lobe torsion after right upper lobectomy is most common ( Fig. 68.10 ).
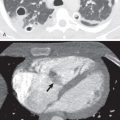
Cardiac herniation is an extremely rare but potentially lethal complication after pneumonectomy, with an intrapericardial approach in which the pericardial defect is not closed adequately. The heart herniates through this defect into the empty hemithorax ( Fig. 68.11 ). It occurs within the first 24 hours postoperatively. Without prompt recognition, the mortality rate is nearly 50%. Chest radiography is diagnostic of right-sided herniation, demonstrating displacement of the heart into the right side of the chest. An early sign of right-sided herniation is a focal convex bulge of the right border of the heart, representing early herniation of the right atrium, termed the snow cone sign. In contrast, left-sided herniation is difficult to diagnose on the posteroanterior radiograph, and the only reliable finding for diagnosis is a posteriorly displaced heart on the lateral chest radiograph. Cardiac herniation is easily recognized on CT by shift of the heart into the hemithorax.

Lung Transplantation
Description
Lung transplantation involves extraction of the donor lung, resection of the recipient’s lung, and implantation of the donor lung. Extraction of the donor lung involves separation of pleural attachments, harvesting of the pulmonary veins along with a left atrial cuff, and division of the pulmonary bronchus and artery. Unlike other pneumonectomies, transplant recipient pneumonectomy is performed with a more distal ligation of the pulmonary artery, immediately beyond the first upper lobe branch. Implantation comprises three anastomoses performed in the posterior-to-anterior anatomic order: bronchus, pulmonary artery, and pulmonary veins-left atrium. Flaps of intercostal muscle, pericardium, or omentum are sometimes used to wrap the end-to-end bronchial anastomoses in an attempt to reduce the incidence of bronchial dehiscence. Some surgeons use a “telescoping” technique when there is bronchial size discrepancy that involves insertion of the smaller bronchus into the larger bronchus, followed by suturing at the external margin of the overlap. When bronchial size is the same, end-to-end anastomosis is performed. Infrequently, in an attempt to reduce the incidence of bronchiolitis obliterans syndrome and bronchial dehiscence, bronchial circulation reconstruction is performed and involves connection of the donor bronchial arteries to the recipient internal thoracic arteries (internal mammary arteries). Single-lung transplantation is typically performed through a posterolateral thoracotomy; bilateral transplantation is performed through a clamshell incision. Living-related lobar transplantation consists of lower lobe harvested from two living related donors, followed by implantation into the respective hemithoraces of a relative, typically a child.
Indications, Contraindications, Purpose, and Underlying Mechanisms
Lung transplantation is a treatment option for severe end-stage pulmonary and vascular diseases, including chronic obstructive pulmonary disease, idiopathic pulmonary fibrosis, cystic fibrosis, primary pulmonary hypertension, and, rarely, pulmonary fibrosis associated with collagen vascular disease, sarcoidosis, lymphangioleiomyomatosis, and pulmonary Langerhans cell histiocytosis. Pulmonary emphysema, including α1-antitrypsin deficiency, is the most common primary disease, accounting for about half of transplantation recipients. Double-lung transplantation is the procedure of choice for cystic fibrosis because of propensity for chronic bacterial pneumonia or colonization.
Expected Appearance on Relevant Modalities
In the early postoperative period, imaging shows chest tubes in both the apex and base, small pleural effusions, and mild pulmonary edema because of the positive fluid balance. At the bronchial anastomotic site, CT often demonstrates a small amount of peribronchial air, and if telescoping has been performed, a small anterior endoluminal flap is also frequently visualized.
Potential Complications and Radiologic Appearance
The diversity of complications of lung transplantation is attributed to the complexity of the surgical technique, the severity and chronicity of the underlying pathology, and the need for immunosuppressive therapy. Complications related to lung transplantation include primary graft dysfunction (PGD), graft rejection, bronchial dehiscence, bronchiolitis obliterans syndrome (BOS), upper lung fibrosis, and posttransplantation lymphoproliferative disorder (PTLD).
Primary graft dysfunction, also known as reimplantation edema, reperfusion edema, and primary graft failure, occurs in 11% to 100% of lung transplantation patients. Because of increased capillary permeability and alveolar damage caused by a combination of pulmonary ischemia, pulmonary denervation, organ preservation, surgical trauma, and lymphatic disruption in the transplanted lung, interstitial and alveolar edema of the allograft occurs when reperfusion begins. PGD is characterized by declining pulmonary function clinically. PGD occurs within the first 48 to 72 hours postoperatively and typically peaks around day 4, slowly resolving within 3 weeks. On radiography PGD commonly manifests with a noncardiogenic pulmonary edema pattern that includes reticulonodular opacities in the perihilar and basilar regions of the middle and lower lungs and may mimic infection or fluid overload. It is therefore a diagnosis of exclusion. On CT septal thickening with patchy or confluent opacities are frequently observed. Although it is a common postoperative finding, reperfusion edema causing complete lung opacification or worsening after 5 days may represent another complication, such as acute rejection or infection, and requires further evaulation.
Acute graft rejection occurs in nearly all transplant patients and is often recurrent. Once diagnosed, it is usually well controlled with corticosteroids, but it may lead to graft failure. Acute rejection occurs within the first week through the first year after transplantation but most often occurs in the first 3 to 6 months. As in reperfusion edema, radiologic findings are nonspecific, and timing of presentation postoperatively is important for diagnosis. The most common radiologic findings are ground-glass and interstitial opacities, pleural effusions, septal thickening, and “perihilar haze” or “flare.” These changes occur primarily in the middle and lower lobes. However, up to 50% of patients have no radiologic findings, and it is difficult to differentiate acute rejection from other pathologic processes, particularly infection. Therefore transbronchial biopsy is the gold standard for diagnosis.
Airway anastomotic complications include bronchial dehiscence, bronchial stenosis, bronchomalacia, as well as bronchovascular, bronchopleural, and bronchomediastinal fistula. These complications occur in approximately 15% of patients after lung transplantation. Chest radiography is of limited value in identifying bronchial anastomotic complications, but unexplained pneumothorax or pneumomediastinum may suggest bronchial dehiscence. Dehiscence is an early complication, typically occurring 2 to 4 weeks postoperatively; stricture and bronchomalacia are late complications typically occurring 2 to 9 months postoperatively. Risk factors for bronchial anastomotic dehiscence include donor bronchi ischemia, postoperative infection, acute rejection, prolonged mechanical ventilation, and donor-recipient bronchial size discrepancy. Dehiscence is primarily due to local ischemia caused by a combination of bronchial blood supply disruption and tension on the bronchial suture line. On CT a bronchial wall defect at the anastomotic site is the most sensitive and specific sign of dehiscence. Diagnosis is facilitated by multiplanar reconstructions and minimum-intensity projection images. Although perianastomotic air is often present in the early postoperative period, new air or an increase in air is a sensitive sign of dehiscence, especially if it is localized to the omental flap. In addition, the presence of a posterior endoluminal flap should raise the possibility of dehiscence.
Bronchial stenosis is present in up to 10% of patients and can be demonstrated on CT as bronchial narrowing at the anastomotic site. Bronchial stenosis can also be seen, although less frequently (5% of patients), after pulmonary sleeve wedge resection. Bronchomalacia involves the deterioration of bronchial elastic and connective tissue, resulting in airway collapse on expiration. It is identified on dynamic chest CT by a greater than 50% reduction, with some authors now suggesting a 70% reduction threshold in airway lumen diameter on expiration.
Bronchiolitis obliterans syndrome (BOS) is the hallmark of chronic lung rejection. Developing 6 to 18 months postoperatively, BOS refers to the deterioration of graft function, with a progressive decline in forced expiratory volume in 1 second for which there is no other cause, such as infection, acute rejection, or anastomotic complications. BOS is thought to occur through immunologically mediated injury that begins with lymphocytic bronchiolitis and progresses to cause small airway scarring and obliteration. BOS incidence increases with time after transplantation and with increased episodes of acute rejection and cytomegalovirus infection. BOS occurs in a majority of lung transplant recipients surviving at least 3 years. BOS is the principal factor limiting long-term survival and accounts for 40% and 35% of deaths in adult and pediatric patients, respectively, after lung transplantation. Chest radiography is typically normal early in the disease; but late in the disease, it may show decreased peripheral vascular markings, slight volume loss, and subsegmental atelectasis. On chest CT decrease of lung attenuation and vascularity occurs in more than 80% of patients. Expiratory CT demonstrates air trapping and is useful for the early diagnosis of BOS. Other common CT findings are bronchiectasis and bronchial wall thickening ( Fig. 68.12 ).