CHAPTER 59 Postoperative Imaging of Ischemic Cardiac Disease
Congestive heart failure, the most common admitting diagnosis for patients older than 65 years in the United States,1 continues to increase in incidence and prevalence, with more than 500,000 new cases of chronic heart failure diagnosed yearly. The most common cause of heart failure is ischemic cardiomyopathy; the second most common cause is dilated cardiomyopathy. The diagnosis, management, treatment, and rehabilitation of patients suffering from ischemic heart disease represent a large percentage of health care costs. Current interventions for management and treatment of end-stage ischemic heart disease include aggressive medical management, extracorporeal circulatory support, percutaneous left ventricular assist device placement, implantable ventricular assist device placement, coronary artery revascularization, mitral valve repair or replacement, scar ablation, passive epicardial restraint, surgical ventricular restoration, and heart transplantation. Combinations of these surgical interventions are sometimes used, depending on the patient’s needs.
PREVALENCE, EPIDEMIOLOGY, AND BACKGROUND
For patients obtaining ventricular assist devices as a bridge to transplantation or destination therapy, 1-year survival rates have been reported to be 52% compared with 25% for a medically treated cohort of patients; the 2-year survival rate is 23% compared with 8% for a medically treated cohort of patients.2
In one study including 1198 patients, 5-year survival rates of patients undergoing surgical ventricular restoration with concomitant coronary artery bypass graft or mitral valve surgery were 69.9% ± 4.7% for New York Heart Association (NYHA) class III patients.3 Results of the STICH (Surgical Treatment for Ischemic Heart Failure) trial, a recently completed randomized surgical versus medical trial, will help clarify indications for coronary revascularization, surgical ventricular restoration, and medical therapy. Alternative therapies to heart transplantation will continue to be important because of the yearly increasing gap between demand and supply of hearts.
Surgical Ventricular Restoration: Dor Procedure
The Dor procedure involves excision of akinetic or dyskinetic and nonviable myocardium of the left ventricle and patch repair of the distal left ventricular cavity, thus restoring the normal elliptical shape of the left ventricle from a spherical dilated heart. The opening of the ventricle is closed by Dacron patch or stitches.4 The restoration of ventricular volume reduces the stress on the ventricular wall, reduces myocardial oxygen consumption, and increases wall contractility and compliance.5 Associated mitral valve regurgitation or intraventricular thrombi are corrected simultaneously. Before surgery, appropriate coronary revascularization procedures, including grafting of the left anterior descending coronary artery, which supplies a high portion of the septum, should be performed.5 Perioperatively, appropriate ventricular volume is restored in the septal and anterior wall without deforming the chamber that will result in neither restrictive nor dilated cardiomyopathy. Care is also taken to achieve an optimal postoperative ventricular short-axis/long-axis ratio; otherwise, mitral regurgitation can result.
The first reported surgery to treat left ventricle aneurysms by Cooley and colleagues involved excision of the thinned segment with linear closure of the free edges.6 Alternative approaches were developed by Dor and Jatene; an intraventricular patch was placed to exclude akinetic and nonresectable areas. The Dor procedure, or endoventricular circular patch plasty, was first performed in 1985.4 Some of the criteria for the Dor procedure are ischemic dilated cardiomyopathy involving one third or more of the ventricular perimeter that causes a spherical dilated left ventricle with akinetic or dyskinetic portions of the septum and anterior wall with end-diastolic volume above 100 mL/m2, reduced ejection fraction (<20%), left ventricular regional asynergy (>35%), and symptomatic patient (angina, heart failure, arrhythmias, and inducible ischemia). Contraindications to the procedure are systolic pulmonary artery pressure above 60 mm Hg without associated mitral regurgitation, severe right ventricular dysfunction, and regional asynergy without ventricular dilation.
Cardiac Transplantation
Cardiac transplantation has been established as the most reliable permanent treatment option for patients with deteriorating heart failure due to ischemic dilated cardiomyopathy despite maximum medical therapy and other revascularization techniques. The most commonly performed type is orthotopic cardiac transplantation, in which the recipient heart is removed except for the posterior aspect of the atrial cuffs. The donor heart is then attached to the recipient’s atria, and the donor’s ascending aorta and main pulmonary artery are anastomosed end to end to the severed ends of the recipient’s ascending aorta and main pulmonary artery, respectively.7
Patients with nonischemic causes of heart failure, such as hypertensive heart disease, myocarditis, idiopathic cardiomyopathy, valvular heart disease, congenital heart disease, and peripartum cardiomyopathy, can also benefit from cardiac transplantation.8 Contraindications to heart transplantation may include AIDS, active systemic infection, malignant disease, irreversible pulmonary hypertension, irreversible secondary organ failure, comorbid life-threatening conditions, active substance abuse, psychiatric history likely to result in noncompliance, cachexia or obesity, chronic obstructive pulmonary disease, renal insufficiency, continued smoking, and severe osteoporosis.
Heterotopic cardiac transplantation is performed in patients with potentially reversible cardiac dysfunction, high pulmonary vascular resistance, or small donor hearts.9 The donor heart is placed anterior to the right lung along the right side of the native heart, and the two left atria are anastomosed, resulting in a common left atrium. The orifices of the donor inferior vena cava and right pulmonary veins are closed. The donor ascending aorta is anastomosed to the recipient aorta in end-to-side fashion, and the donor main pulmonary artery is combined with a Dacron graft, resulting in an end-to-side anastomosis with the recipient main pulmonary artery. The donor superior vena cava and right atrium are connected to the native right atrium, allowing systemic venous return to pass into either the native or the donor right ventricle. Chambers involved in functioning include the right ventricle of the recipient and the left ventricle of the donor.
POSTOPERATIVE ASSESSMENT
General Surgery-Related Complications
Mediastinal hematoma, pericardial hematoma, pleural effusions, pneumothorax, hemothorax, pneumoperitoneum and hemoperitoneum, basal lung atelectasis, aspiration, and infectious pneumonia are common complications related to these procedures in the immediate postoperative period. It is not uncommon to identify malpositioning of support tubes and catheters or a foreign body such as a surgical sponge on the portable radiographs. Median sternotomy complications, such as mediastinitis and sternal wound infections, may occur. These patients are prone to pulmonary embolism during the perioperative and immediate postoperative period. Perioperative bleeding is a result of prolonged surgical time under extracorporeal circulation and hypothermia, use of anticoagulants and antiplatelets drugs, vascular injury to the surgical bed, associated malnourishment, and hepatic dysfunction due to low-flow state and congestive hepatopathy.1
Specific Complications
Ventricular Assist Device
Right-sided heart failure may be due to adverse effects of the LVAD on the interventricular septum, increased pulmonary pressure due cardiopulmonary bypass or massive blood transfusions, and right coronary artery disease.1 After the perioperative period, infection related to the VAD, thromboembolism with infarction, and limited reliability of the VAD remain the most important concerns. Patients with a VAD are more susceptible to infection because of tracking along subcutaneous drivelines for connecting batteries and controllers, leading to entry and exit site infection, driveline infection, or pump infection. Pump infection may require removal of the device and hence is of the greatest concern.1 Thrombus can sometimes be seen in the cardiac chambers or inflow and outflow cannulas. Thromboembolism and resultant infarction of lung, brain, or systemic organs may occur after VAD placement, although the HeartMate device is believed to have less chance for this development because of the textured surface of the blood-containing chamber by a polyurethane diaphragm, which leads to pseudointima formation.10 Aortic dissection at the level of the ascending aorta can result from high-velocity blood injected against the aortic wall. Device reliability depends on the type of device; it is 1 to 3 years for pulsatile pumps and about 5 years for miniaturized axial flow pumps. However, because the VAD does not fail catastrophically, further treatment by device exchange or transplantation can be warranted.1
Dor Procedure
Specific complications associated with surgical ventricular restoration are suture line or patch dehiscence, excessive reduction in left ventricular volume resulting in mitral regurgitation, and restrictive or constrictive physiology.11 Intracardiac thrombus that is adherent to the patch at the left ventricle apex is a noted complication of this procedure.
Cardiac Transplantation
Early postoperative complications (between 0 and 30 days) are related to surgical complications, including cardiac ischemia, pulmonary edema, and anoxic brain injury and thromboembolic events. Intermediate postoperative complications (between 1 month and 12 months) mainly include acute allograft rejection and infection. Allograft rejection is manifested usually between 2 and 12 weeks after transplantation.9 The time of greatest immunosuppression is during the first 3 months after transplantation. Bacterial (predominantly aerobic gram-negative rods), viral, and fungal infections occur within the first month and often affect the lungs.
Mediastinal infection can lead to weakening of suture lines and cause aortic dissection and pseudoaneurysm formation. Late complications (after 12 months) resulting in death include transplant-associated coronary artery accelerated graft atherosclerosis, malignant disease, infection, transplant rejection, aortic allograft rejection, and cerebral infarctions.12
Coronary allograft vasculopathy or atherosclerosis is caused by immune-mediated and nonimmunologic injury,7 which affects half of patients within 5 years of transplantation and is characterized by diffuse concentric intimal thickening of both proximal and distal coronary arteries. The major risk factors for late mortality from coronary allograft vasculopathy include ischemic heart disease, younger recipient age (but older than 20 years), black race, cigarette use within 6 months of listing for transplantation, older donor age, and development of coronary artery disease during the first post-transplant year.13 Malignant neoplasms are likely to be secondary to long-term immunosuppression and include lymphomas, acute leukemia, visceral tumors, Kaposi sarcoma, gynecologic cancers, primary lung carcinoma, skin cancer (predominantly squamous cell cancer), and post-transplant lymphoproliferative disorder.9
Other complications are related to long-term corticosteroid use and immunosuppression. These include osteoporosis, vertebral insufficiency fractures, and lipomatosis.8
CLINICAL PRESENTATION
Patients with mediastinitis are often unwell and may have fever, tachycardia, chest discomfort, sternal tenderness, and leukocytosis. Discharge from the sternotomy wound with delayed healing may be indirect evidence of ongoing sternal osteomyelitis or a mediastinal infection or collection.9
Fever or lethargy may be the first indicator of internal systemic infection. If it is associated with breathlessness, productive cough, and pleuritic chest pain, it can be a warning symptom of community-acquired or opportunistic pulmonary infections, which are quite common in the postoperative period because of immunosuppression. Patients with infective (mycotic) pseudoaneurysms may be asymptomatic or may present with fever, lethargy, and chest pain.9 Pulmonary embolism is typically manifested with sudden-onset breathlessness, chest pain, tachycardia, and hypoxia.
In heart transplantation, a common complication in the postoperative period is impending rejection, which may be asymptomatic or can be manifested by dyspnea, palpitation, fatigability, weakness, syncope and signs suggestive of hypotension, worsening of cardiac function, and increasing heart failure.9
Clinical manifestations of coronary vasculopathy include myocardial infarction, graft failure, arrhythmias, and sudden death. It starts distally in small coronaries and progresses proximally to epicardial vessels without formation of collaterals.7 Because of the difficulty in preventing this complication and the clinically silent presentation, routine surveillance has been advocated for detection and early intervention by revascularization procedures.7
New-onset back pain in patients receiving corticosteroids should suggest osteoporosis, vertebral fractures, intervertebral diskitis, or internal malignant disease with bone metastases. Gastrointestinal complications, such as peptic ulcer, diverticulitis, and organ perforation, may go unnoticed because of the effect of corticosteroids, which may mask signs and symptoms.8 A high index of clinical suspicion and appropriate timely imaging would help in identifying these serious pathologic processes.
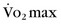 of less than 14 mL/kg per minute,
of less than 14 mL/kg per minute,


