Cancers of the pharynx and larynx are treated using a combination of chemotherapeutic, radiation, and surgical techniques, depending on the cancer type, biology, location, and stage, as well as patient and other factors. When imaging in the postsurgical setting, the knowledge of the type of tumor, preoperative appearance, and type of surgery performed is essential for accurate interpretation. Surgical anatomic changes, surgical implants/devices, and potential postsurgical complications must be differentiated from suspected recurrent tumors.
Key points
- •
The complex functional anatomy of the pharynx and larynx necessitates multiple surgical techniques depending on tumor location and extent.
- •
Knowledge of surgical approaches to laryngeal and pharyngeal cancers is key to differentiating expected post-operative changes from complications.
- •
Post-surgical changes may confound interpretation when evaluating for recurrent disease, and common false-positive and false-negative appearances should be understood.
Introduction
Cancers of the pharynx and larynx comprise 3.5% of all newly diagnosed cancers in the United States with more than 66,000 new cases each year and almost 4000 deaths. Most of these, more than 95%, are squamous cell carcinomas. These cancers are treated using a combination of chemotherapeutic, radiation, and surgical techniques, depending on the cancer type, biology, location, and stage, as well as patient and other factors. When imaging in the postsurgical setting, the knowledge of the type of tumor, preoperative appearance, and type of surgery performed is essential for accurate interpretation. Surgical anatomic changes, surgical implants/devices, and potential postsurgical complications must be differentiated from suspected recurrent tumors.
Imaging protocols
Cross-sectional CT, PET, and MRI are the mainstays of pharynx and larynx cancer diagnostic and surveillance imaging. After definitive therapy, whether surgical or nonsurgical, imaging is performed primarily to evaluate local persistent or recurrent disease as well as regional or distant metastasis. Imaging may also be performed to evaluate for posttreatment complications, and the modality and protocol should be tailored to the specific indication.
Surveillance imaging is primarily performed with CT, MRI, combined PET/CT or PET/MRI, and ultrasound. CT, MRI, and PET are the mainstays, with a relatively high sensitivity ranging from 50% to 100% and specificity ranging from 33% to 100%. , PET has been shown to have the highest sensitivity for primary and recurrent disease, and as a result, is the modality of choice for most referring clinicians for surveillance. Surveillance imaging timelines and algorithms vary widely among radiation oncologists and surgeons, and recommendations vary depending on tumor location, size, and presence of regional metastases. Most patients will undergo imaging within 6 months posttreatment; however, the benefit of continued surveillance imaging beyond 6 months in asymptomatic patients is unclear. , The National Comprehensive Cancer Network guidelines have no specific imaging follow-up recommendations beyond 6 months posttreatment in asymptomatic patients. The American College of Radiology (ACR) NI-RADS whitepaper recommends an extended timeline to approximately 2 years posttreatment, a period during which 95% of asymptomatic recurrences are detected. The ACR surveillance algorithm includes PET/CT at 8 to 12 months after the completion of definitive therapy for baseline, CT, or PET/CT 6 months later if negative, CT alone 6 months later if negative, and finally, if imaging remains negative, CT 12 months later. The use of this extended algorithm has shown the ability to detect recurrences beyond 6 months and before clinical signs are evident in 81% of asymptomatic patients.
In symptomatic patients, in the setting of a suspected surgical complication, or suspected recurrence, imaging should be tailored to the clinical scenario. If there is suspicion for recurrent disease, PET/CT or PET/MRI should be considered for greatest sensitivity for active disease and to provide both functional and anatomic detail. If there is a suspected surgical complication such as infection or hemorrhage, CT, MRI, or ultrasound may be preferred to evaluate anatomy without the added radiation dose and time conferred by a PET examination.
Expected postoperative findings of the pharynx and larynx
Nasopharyngectomy
Nasopharyngectomy is most often used as a salvage treatment for recurrent or residual nasopharyngeal carcinoma (NPC) after definitive radiation therapy. Tumors will recur either locally or regionally in approximately 10% of patients, and in these radioresistant tumors, surgery is often recommended. NPC is uncommon cancer, representing 0.7% of all cancers diagnosed in 2018, with more than 70% of cases occurring in east and southeast Asia, and showing an association with Epstein–Barr virus infection.
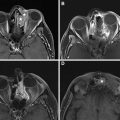
Salvage surgical resection of recurrent NPC results in 5-year survival of 30% to 52%, and gives less morbidity than reirradiation. Given the deep location of the nasopharynx and many high-risk structures in close proximity, surgical access presents a challenge. Traditional open approaches provide excellent access to and visualization of tumors; however, they are technically complicated and associated with a relatively high risk of morbidity, including nerve injury, cosmetic and functional deficits, and vascular injury, among others. , , Minimally invasive endoscopic techniques have been developed to help mitigate these issues, including transnasal and transoral approaches , , ( Fig. 1 ).
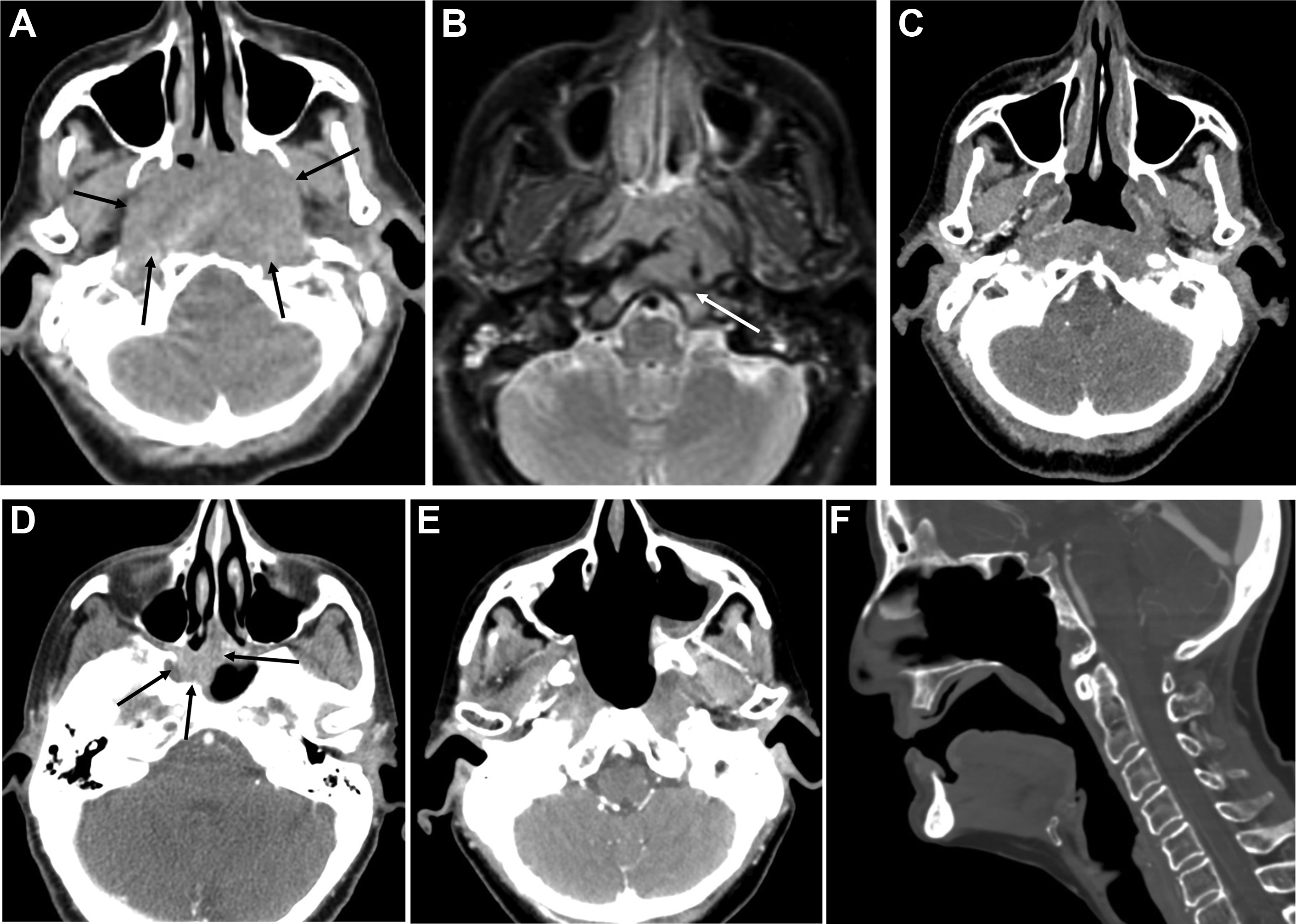
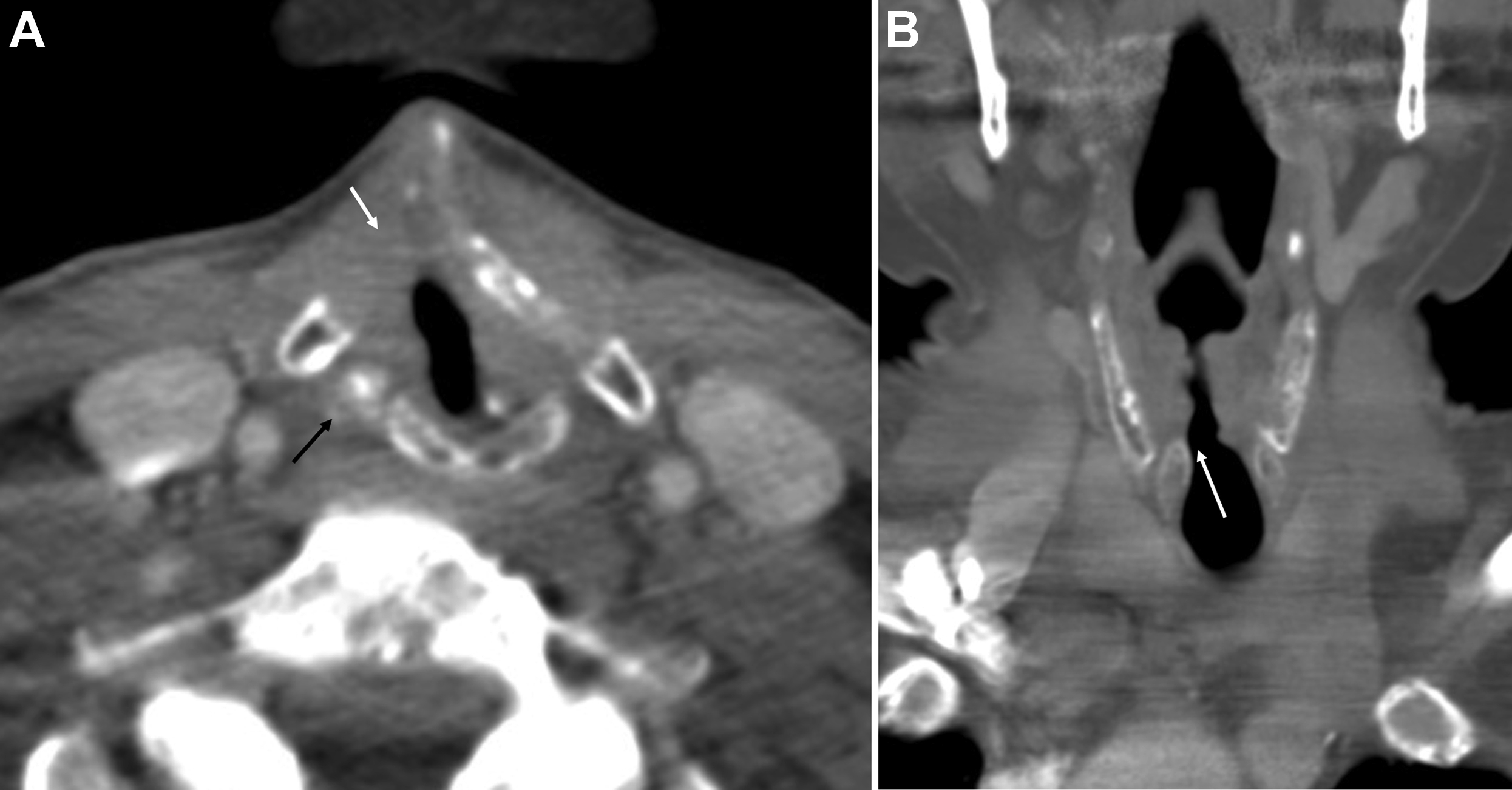
For a transnasal endoscopic approach, operative exposure of the tumor is important and may be maximized by the removal of the posterior bony septum (vomer) and inferior turbinates. More extensive exposure by ethmoidectomy, middle and superior turbinectomies, and medial maxillectomy may be performed depending on the tumor extent. , For small centrally located tumors limited to the posterior nasopharyngeal wall, the resection can spare the Eustachian tube laterally and extend posteriorly through the longus capitis muscles to the ventral clivus periosteum with the drilling of the clivus. For tumors that extend to the roof of the nasopharynx, the resection may include the floor and anterior wall of the sphenoid sinus as well as the superior turbinates. For tumors that extend laterally to obtain adequate exposure a complete ethmoidectomy, medial maxillectomy (possibly including the lateral pyriform aperture), unroofing of the pterygopalatine fossa, and removal of the medial pterygoid plate may be performed. The resection then may include the lateral nasopharyngeal wall with the removal of the cartilaginous Eustachian tube up to the bony junction. , The surgical resection can also be extended to the middle cranial fossa if necessary. In the event of tumor involvement of the parapharyngeal internal carotid artery (ICA), the ICA may also be partially resected endoscopically provided that the ICA was preoperatively sacrificed. Surgical defects of the middle or posterior cranial fossae, or exposed ICA may be covered with a nasoseptal mucoperiosteal flap, a tunneled temporoparietal fascia flap, or free allograft. ,
When tumors extend beyond the field easily exposed and accessed via an endoscopic transnasal approach, for example, inferior to the plane of the hard palate in the parapharyngeal space, laterally into the infratemporal fossa beyond the pterygoid musculature, or posterior–inferior involvement of the ICA, an open transmaxillary or infratemporal fossa approach may be necessary for adequate visualization. More recently, an endoscopic transoral approach has been described for these tumors, allowing adequate visualization of more inferiorly and laterally extensive tumors by improving cosmesis and morbidity than open approaches.
As the operative approach and resection extent can vary dramatically, comparison with preoperative imaging is critical when interpreting postoperative imaging studies. In general, expected findings in the immediate postoperative setting will include nonmass-like enhancement at the margins of the resection, with or without blood, gas, and surgical material. As these operations are most often performed in the setting of prior radiation therapy, a background of postradiation changes will often be seen, including poor delineation of normal fat planes, fibrosis, ill-defined enhancement, and bone marrow edema or fatty replacement. Postoperative enhancement and marrow edema may be difficult to differentiate from postradiation changes, previously treated nonviable tumor, or residual viable tumor particularly in the parapharyngeal space, pterygopalatine fossa, orbits, and skull base. Comparison with preoperative imaging, stability over time, and concurrent PET/CT or PET/MRI is very helpful in distinguishing posttreatment changes from viable tumor as further described in later sections.
Laryngectomy
Laryngeal cancers comprise approximately 20% of all head and neck cancers, with the vast majority being squamous cell carcinomas. Treatment may involve any combination of chemotherapy, radiotherapy, and surgery, depending on tumor and patient characteristics. For surgical management, the type of resection depends on the extent of the primary tumor as well as the presence and extent of regional or distant metastases ( Table 1 ).
| Laryngectomy Type | Indications | Contraindications |
|---|---|---|
| Endoscopic Cordectomy | Unilateral true vocal cord lesion | Spread outside of true cord, that is, the anterior commissure, paraglottic space, arytenoid cartilage |
| Vertical Partial Laryngectomy | True cord lesion involving anterior commissure or arytenoid cartilage | Invasion of the thyroid cartilage or posterior commissure, cord fixation, or transglottic spread |
| Supraglottic Laryngectomy | Supraglottic lesion with normal cord mobility and no involvement of the ventricles | Spread to the glottis, thyroid cartilage, cricoid cartilage, or postcricoid area, cord fixation, spread to the base of tongue or apex of the pyriform sinus |
| Supracricoid Laryngectomy | Supraglottic, glottic, or transglottic lesions with cord fixation or other contraindications to partial or supraglottic laryngectomy | Invasion of the hyoid, arytenoid cartilage fixation, extension to subglottic larynx or base of tongue, massive preepiglottic or paraglottic space invasion, invasion of thyroid cartilage outer perichondrium, extralaryngeal spread of tumor |
| Near Total Laryngectomy | Unilateral laryngeal or pyriform sinus lesion with the involvement of the cricoid cartilage | Invasion of the interarytenoid or postcricoid regions, involvement of bilateral vocal cord, and arytenoid involvement |
| Total Laryngectomy | Extensive laryngeal tumors with cartilage invasion, cricoid involvement, salvage surgery after failed radiation or partial laryngectomy | Metastasis, synchronous tumor, invasion of the prevertebral fascia, encasement of the common or internal carotid arteries |
The larynx is subdivided into 3 regions: the supraglottic, glottic, and subglottic larynx. The supraglottic larynx is bordered superiorly by the lingual surface of the epiglottis and the hyoepiglottic ligament, laterally by the laryngeal surfaces of the aryepiglottic folds, anteriorly by the thyrohyoid ligament and inferiorly by the laryngeal ventricles. The glottis extends from the laryngeal ventricles superiorly to include the true vocal cords (comprised of the epithelium, superficial lamina propria, vocal ligament, and vocalis muscle), the anterior commissure, the vocal processes of the arytenoid cartilages, and ending 1 cm below the plane of the vocal cords. The subglottic larynx extends inferiorly from the glottis to the inferior border of the cricoid cartilage. The interarytenoid space and posterior wall of the cricoid cartilage form the posterior border of the glottic and subglottic larynx, whereas the thyroid cartilage, cricoid cartilage, and cricothyroid membrane form the anterior border. ,
The goal of surgery in laryngeal cancer is to remove the tumor by preserving as much function of the larynx and pharynx as possible. Multiple techniques may be used depending on tumor extent to maximize functional and oncologic outcomes.
Partial laryngectomy
For small tumors contained to the true vocal cord unilaterally, without the involvement of the anterior commissure or paraglottic space, and without the impairment of vocal cord mobility, endoscopic cordectomy with carbon dioxide laser may be performed. The true vocal cord and vocalis muscle only are resected, and after healing and regeneration of the vocal cord, imaging may seem normal.
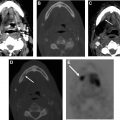
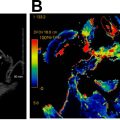
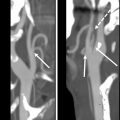
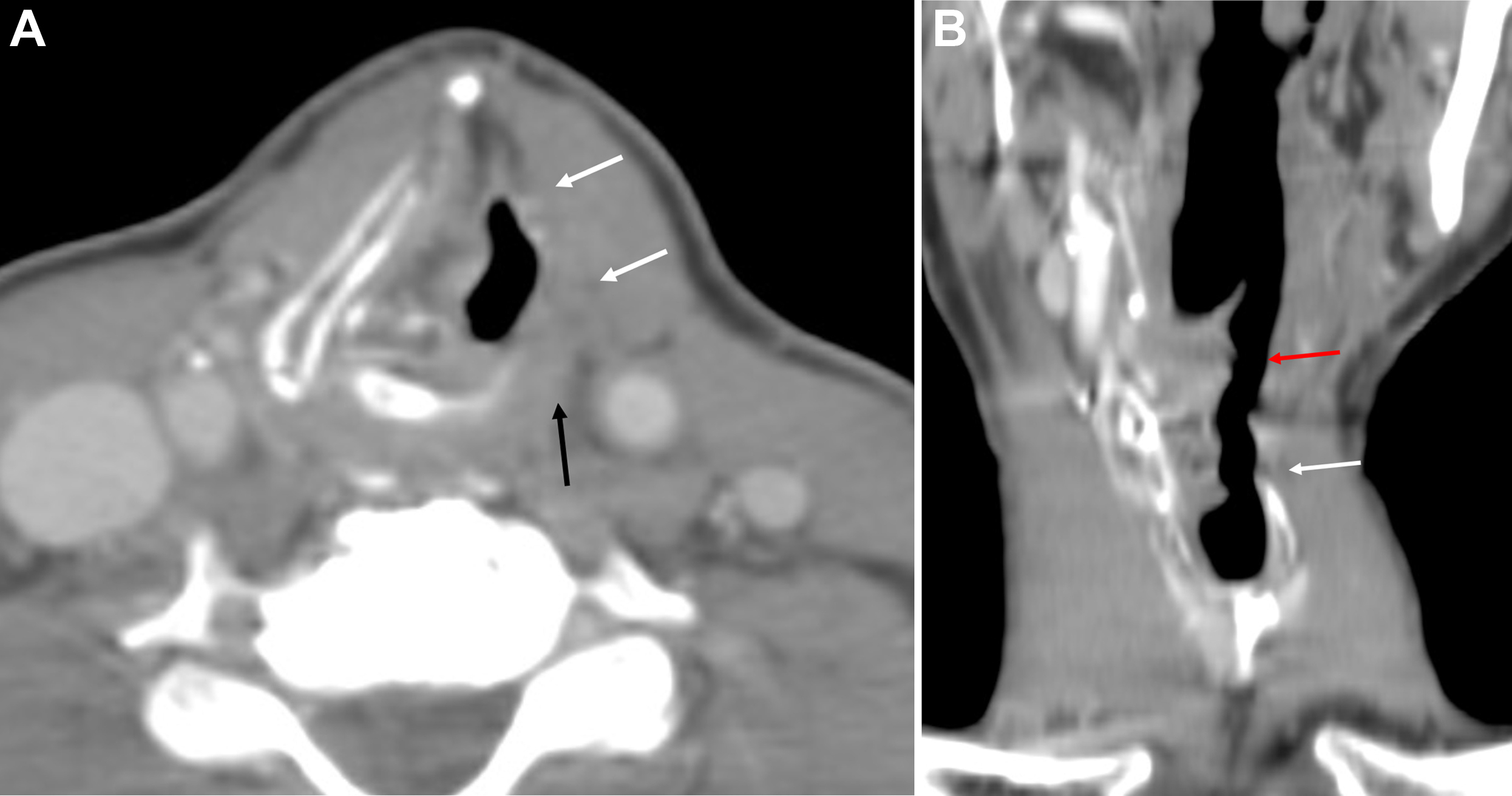
For tumors that involve either the anterior commissure or arytenoid cartilage, vertical partial laryngectomy may be performed either by frontolateral partial laryngectomy or hemilaryngectomy. In frontolateral partial laryngectomy, a vertical segment of the thyroid cartilage anterior to the tumor is removed, and the involved vocal cord, ipsilateral laryngeal ventricle and false cord, anterior commissure, and anterior-most portion of the contralateral vocal cord are removed ( Fig. 2 ). The ipsilateral arytenoid cartilage may or may not be removed. The contralateral vocal cord mucosa is repaired primarily and the ipsilateral resection site heals by secondary intention. In hemilaryngectomy, the ipsilateral thyroid cartilage lamina is removed along with the ipsilateral true vocal cord up to one-third of the anterior contralateral vocal cord, the ipsilateral arytenoid cartilage, and the mucosa of the ipsilateral aryepiglottic fold ( Fig. 3 ). Postoperative imaging may show scarring of the resected vocal cord and paraglottic space resulting in a dense, “pseudo-cord” appearance, as well as surgical defects of the thyroid lamina and absence of the ipsilateral arytenoid cartilage. ,
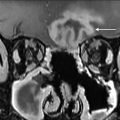
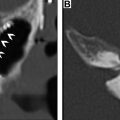
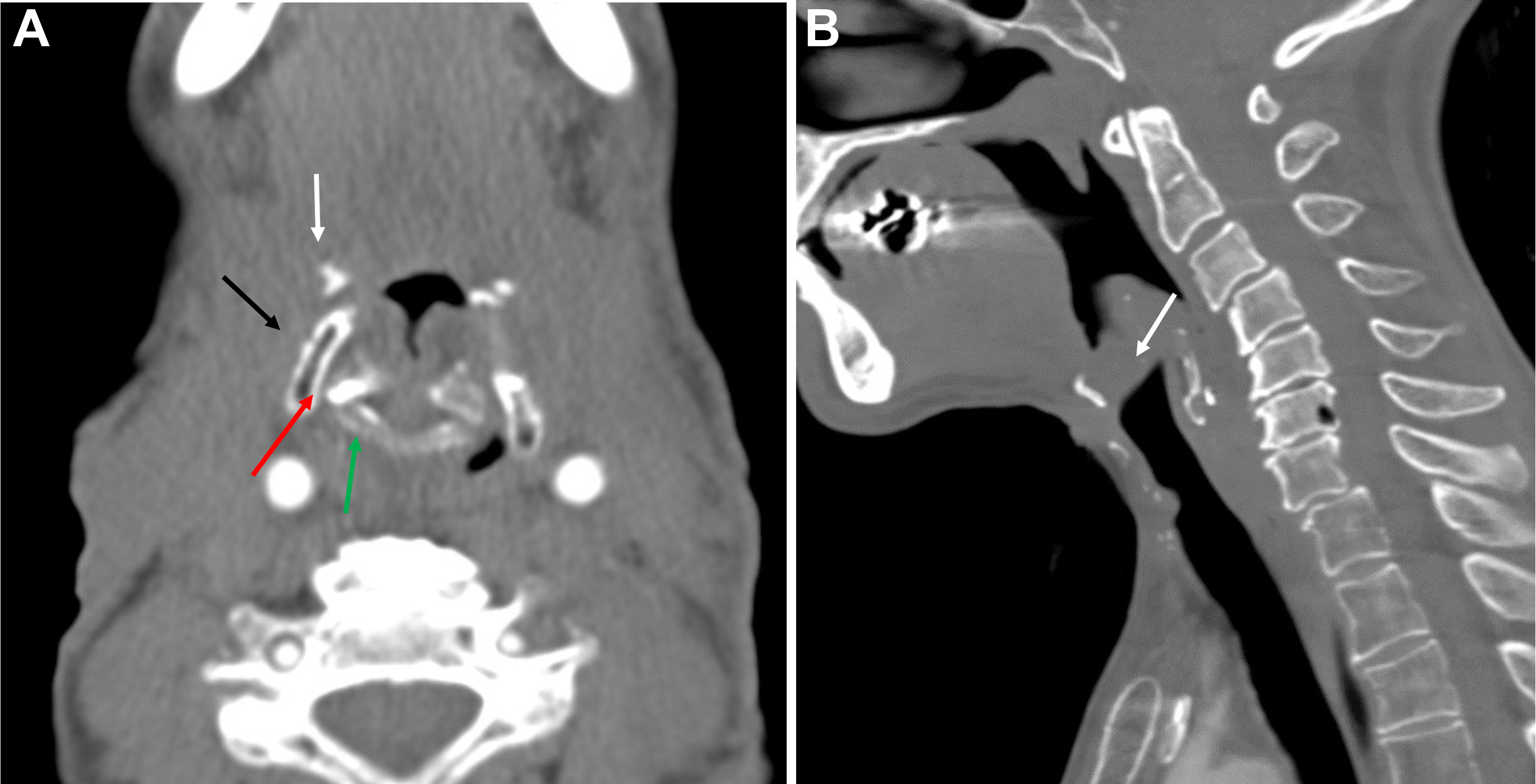
For supraglottic laryngeal cancers in which there is no involvement of the glottis, laryngeal ventricles, cartilage invasion, pyriform sinus apex, or base of tongue, a supraglottic laryngectomy may be performed. This procedure involves the removal of the epiglottis, aryepiglottic folds, false vocal cords, and laryngeal ventricles, as well as the upper third of the thyroid cartilage and thyrohyoid membrane. A thyrohyoidopexy is then performed, raising the remaining lower two-thirds of the thyroid cartilage and attaching it to the underside of the hyoid. Postoperative imaging will show the foreshortened larynx, absence of the epiglottis, an air-filled cavity in the supraglottic region, and approximation of the hyoid and remaining thyroid cartilage with an otherwise normal-appearing glottic and subglottic larynx ( Fig. 4 ). , Variations of the supraglottic laryngectomy include the extended supraglottic laryngectomy with the removal of one arytenoid cartilage, the base of the tongue, or the pyriform sinus, and the three-quarters laryngectomy with the removal of the ipsilateral true vocal cord and arytenoid cartilage in addition to the usual supraglottic structures in the event of spread to the glottis on one side, provided there is no cord fixation or cartilage invasion.

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree