Chapter Outline
Diagnostic Evaluation Following Gastric and Duodenal Surgery
Surgical Considerations for Peptic Ulcer Disease
Surgical Procedures for Gastric Resection
Surgical Considerations for Gastric and Duodenal Neoplasms
Gastric and duodenal operations are usually performed for peptic ulcer disease (PUD), benign or malignant gastric or duodenum masses, and the treatment of obesity. Imaging studies are often an integral part of the evaluation of patients following gastric or duodenal surgery. Radiologic evaluation of postoperative patients requires understanding of the operative procedures, appreciation of the expected postoperative appearance, and knowledge of potential complications. In the early postoperative setting, the radiologist is often asked to assess the postsurgical anatomy and evaluate for possible early postoperative complications such as leakage. In the late postoperative course, radiologic evaluation may be undertaken to evaluate for more delayed complications or symptoms of abdominal pain, nausea and vomiting, weight loss, and weight gain.
Gastroduodenal surgical procedures may include resection and/or bypass of portions of the intestinal tract. Treatment of PUD may involve some form of vagotomy, often in conjunction with a procedure to improve gastric emptying (pyloroplasty or antrectomy). Following distal gastric resection, continuity of the gastrointestinal (GI) tract may be reestablished via a Billroth I gastroduodenostomy or gastrojejunostomy (Billroth II or Roux-en-Y gastrojejunostomy). For treatment of gastric neoplasms, gastrojejunostomy is favored over gastroduodenostomy because this allows for wider surgical margins. The most common bariatric procedures currently performed in the United States include Roux-en-Y gastric bypass, laparoscopic adjustable gastric banding, and sleeve gastrectomy (gastric sleeve).
This chapter provides an overview of the more common surgical procedures performed on the stomach and duodenum as well as the indications for surgery, approaches to the radiologic evaluation, expected postsurgical appearance, and possible complications. Radiologists need to have an understanding of the expected postoperative anatomy to assess for possible complications and diagnose recurrent disease.
Diagnostic Evaluation Following Gastric and Duodenal Surgery
The imaging approach in patients following gastric and duodenal surgery depends on the type of surgical procedure performed, postoperative time course, patient’s clinical status, and patient’s presenting symptoms. Fluoroscopic and computed tomography (CT) studies are usually performed to evaluate postoperative patients; both may occasionally be necessary to establish the most accurate diagnosis.
Fluoroscopic Studies and Upper Gastrointestinal Examinations.
Following abdominal surgery, an overhead scout radiograph should be obtained prior to administration of contrast to assess for postoperative clips, a staples and/or suture lines. The pattern of sutures and staples may help determine the surgical procedure performed if the patient history is unreliable. Also, surgical material should be identified prior to the administration of oral contrast so that radiopaque surgical material is not later mistaken for a small leak. In the early postoperative course, drains and abnormal gas collections may be assessed. Any expected or inadvertent indwelling surgical foreign body (e.g., gastric band device) should also be assessed on the scout radiograph.
In patients following gastric and/or duodenal surgery, the postoperative anatomy should be evaluated at the onset of the fluoroscopic procedure. Because each patient has unique surgical variations, a detailed, cookbook-style upper gastrointestinal examination (UGI) protocol for position changes during the study and required exposures does not tend to apply. Studies must be modified based on the surgical anatomy. Optimal patient positioning to evaluate an anastomosis is variable, depending on the type of surgical procedure performed. The postoperative anatomy involving the stomach or duodenum should be evaluated prior to proceeding to other portions of the planned examination (e.g., assessment of esophageal motility or esophageal pathology). The initial contrast bolus should be observed fluoroscopically through the resected, oversewn, banded, sutured, or stapled sites and promptly imaged before there is contrast opacification of overlapping structures.
In the early postoperative setting, a UGI should be performed initially using water-soluble contrast agents to assess for possible postoperative leak because water-soluble contrast agents have no known damaging effects when extravasated into the peritoneal cavity or mediastinum. If no leak is identified with water-soluble contrast, barium should be administered. Barium may detect more subtle leaks and other complications. If the study is performed in the late postoperative course and a leak is not suspected, barium may be initially administered.
Single-contrast technique may be preferable to assess the postoperative anatomy, including any surgical anastomosis, and to evaluate for possible fistulas or staple line dehiscence following gastric surgery. In addition, supine patient positioning may be necessary and preferable in the debilitated or early postoperative patient. Double-contrast studies may provide more optimal assessment of mucosal detail, but may be difficult to perform in the postoperative patient because of diminished patient mobility or cooperation. Also, a patulous gastrojejunal or gastroduodenal anastomosis (gastric outlet) may preclude optimal gaseous distention of a gastric pouch or remnant. Intravenous (IV) glucagon administration may help slow the passage of barium and air through the gastric remnant and therefore allow for more optimal distention.
Computed Tomography.
Computed tomography (CT) may also be performed to assess for postoperative complications. CT can provide detail about the overall anatomy in the abdomen and pelvis, evaluate the bowel in its entirety, and assess the extent of any postoperative fluid collections.
Abdominopelvic CT following gastric and duodenal surgery is ideally performed following the administration of positive oral and IV contrast. Positive oral contrast helps depict the proximal GI anatomy on CT. As with fluoroscopic studies, water-soluble oral contrast should be administered rather than barium in the early postoperative course or if perforation is a consideration. Oral contrast may be administered at intervals prior to the CT study; however, it should also be administered immediately prior to image acquisition to opacify the residual stomach.
Nuclear Medicine.
Scintigraphic studies may be of benefit for patients following gastric and/or duodenal surgery to assess for possible motility problems or postgastrectomy syndromes, including GI stasis and dumping syndrome. Radiolabeled solids or liquids can be used to evaluate gastric emptying. A time-activity curve can be applied to determine the emptying time of the stomach.
Endoscopy.
Endoscopy may be useful to assess a surgical anastomosis and evaluate for possible complications such as marginal ulcer disease. Endoscopy may be of particular benefit for diagnosing inflammatory conditions such as bile reflux gastritis and for diagnosing early tumor recurrence.
Surgical Considerations for Peptic Ulcer Disease
There has been a decline in the number of operative procedures performed for peptic ulcer disease over the last 3 decades. This is partly because of increased use of medications such as histamine-2 blockers (H2 blockers), proton pump inhibitors (PPIs), and agents to protect the gastric mucosa, including sucralfate and prostaglandin analogues. Identification and treatment of Helicobacter pylori infection has been overwhelmingly beneficial, as has the understanding of the role of nonsteroidal anti-inflammatory drugs (NSAIDs) in peptic ulcer disease. Surgical therapy is usually reserved for patients with severe and emergent complications of ulcer disease. The most common complications of peptic disease, in decreasing order of frequency, are bleeding, perforation, and obstruction. Most peptic ulcer related deaths in the United States are caused by bleeding, which may include melena and/or hematemesis. Patients with perforation may present with an acute abdomen. A detailed discussion of peptic ulcer disease and its manifestations may be found elsewhere (see Chapter 29 ).
Indications for Surgery
Indications for surgery in the setting of peptic ulcer disease may include perforation, bleeding, gastric or duodenal obstruction, intractability, and nonhealing ulcers.
Perforation.
In patients with acute ulcer perforation, the ulcer may simply be oversewn. In younger patients in particular, simple closure is often successful, with less than 20% chance of recurrence. However, a simple patch may be inadequate for older patients, and more definitive therapy may entail resection.
Bleeding.
In approximately 70% of cases of major bleeding from PUD, the bleeding stops spontaneously. Surgery may be necessary because of shock or continued bleeding, despite optimal medical management and endoscopic therapy. Surgical management may consist of vagotomy with pyloroplasty or vagotomy with antrectomy (see later).
Obstruction.
Gastric outlet or duodenal obstruction occurs in less than 10% of patients with PUD. This typically requires resection, usually with a gastrojejunostomy.
Nonhealing Ulcers.
Patients with ulcers that fail to heal despite 12 to 15 weeks of optimal medication may be surgical candidates. As many as 10% to 15% of duodenal ulcers do not heal after a 6-week course of high-dose H2 blockers and PPIs, and in 15% to 30% of ulcers that initially heal with medical treatment, relapse occurs. In the case of refractory gastric ulcers, underlying gastric cancer must always be a consideration. In patients with intractable ulcers, elective surgical intervention is preferable, with a lower recurrence rate as compared with more urgent or emergent surgical management.
Surgical Procedures for Peptic Ulcer Disease
The physiologic basis of surgery for PUD includes methods to decrease secretion of hydrochloric acid from parietal cells located in the body and fundus of the stomach and to remove the portion of the gastric mucosa that is most predisposed to ulcers. Typically, surgical management of peptic ulcer disease includes truncal vagotomy with pyloroplasty, vagotomy with distal gastrectomy, or a highly selective vagotomy (HSV). Overall, vagotomy with distal gastrectomy results in the lowest ulcer recurrence rates; however, this has increased morbidity as compared with other treatment options.
Patients undergoing surgery for peptic ulcer disease may alternatively undergo simple oversewing of a bleeding ulcer or a simple patch of a perforated ulcer. UGI may be performed following this surgery to assess for leaks. A simple patch of a perforated ulcer may appear as a localized filling defect or may simulate an ulcer on UGI.
Vagotomy
Vagotomy decreases total acid secretion. Also, parietal cell response to gastrin and other stimulants decreases after vagotomy.
Truncal vagotomy requires complete transection of the anterior and posterior vagus nerve trunk and results in denervation of the stomach, liver, gallbladder, pancreas, small intestines, and proximal large intestine. Loss of the antropyloric mechanism results in gastric stasis, and a pyloroplasty, gastroduodenostomy, or gastrojejunostomy must be performed to improve gastric drainage. Gastrojejunostomy is the preferred choice for patients with gastric outlet obstruction or a severely diseased duodenum. Truncal vagotomy has the side effects of dumping or diarrhea in 10% of patients.
Highly selective vagotomy (HSV), also called parietal cell vagotomy or proximal gastric vagotomy, is a relatively safe procedure with minimal side effects that decreases total acid secretion by 75%. With HSV, the vagal nerve supply to the proximal 2/3 to 3/4 of the stomach (parietal cell location) is severed with preservation of the distal branches to the antrum and pylorus. The antropyloric mechanism remains intact and the innervation to remaining abdominal viscera is preserved, minimizing gastrointestinal side effects. This operation does not alter gastric emptying and therefore does not require a drainage procedure, gastric resection, or an anastomosis. Elective HSV may be a preferred treatment for refractory PUD in the absence of gastric outlet obstruction. However, overall elective HSV for treatment of PUD has largely been replaced by long-term PPI treatment. Also, patients with pyloric and prepyloric ulcers have higher ulcer recurrence rates after HSV than those with duodenal ulcers alone. In this setting, a truncal vagotomy with antrectomy may be preferable.
Radiography or CT studies following vagotomy may reveal multiple clips in the expected distribution of the vagus nerve and/or its branches.
Pyloroplasty
Pyloroplasty consists of reconstructing the pyloric channel to increase its diameter and improve gastric emptying. Following truncal vagotomy, impairment of gastric tone and interruption of the antropyloric mechanism result in gastric stasis, and a gastric drainage procedure is required. Pyloroplasty is relatively easy for the surgeon to perform, with less necessary dissection as compared with antrectomy. It also avoids surgical difficulties that may arise with a duodenal stump following antrectromy, and continuity of the GI tract is not altered.
Heineke-Mikulicz Pyloroplasty.
A Heineke-Mikulicz pyloroplasty consists of a longitudinal incision from the distal antrum to the proximal duodenum through the pylorus, followed by a transverse closure (essentially parallel to the base of the duodenal bulb) to increase the diameter of the pyloric channel.
Finney Pyloroplasty.
A Finney pyloroplasty may be preferred when a longer incision of the duodenum is required to control bleeding or with scarring of the pylorus and duodenal bulb. A Finney pyloroplasty is essentially a side-to-side gastroduodenostomy. An incision is made along the greater curvature of the distal stomach, extending through the pylorus along the medial aspect of the duodenum. An inverted U-shaped incision is made, opening the pyloric channel.
Jaboulay Pyloroplasty.
A Jaboulay pyloroplasty is actually a gastroduodenostomy between the antrum and duodenum. The pylorus is not technically incised. This results in a large opening between the distal stomach and duodenum and avoids any inflammatory process in the pyloric area.
Antrectomy
An antrectomy will remove the source of gastrin and prevent gastric stasis. When performed in combination with truncal vagotomy, antrectomy is the gold standard for reducing acid secretion, with the lowest recurrence rates when compared to vagotomy with pyloroplasty and HSV alone. Antral resection with vagotomy is the most definitive operation for treating ulcer disease. Ulcer recurrence following antrectomy is less than 1%, whereas following vagotomy and pyloroplasty, recurrence rates range from 4% to 27%, and recurrence rates following HSV range from 4% to 11%. However, low recurrence rates must be weighed against postgastrectomy and postvagotomy complications that occur in up to 20% of patients; these may include diarrhea, dumping, bile reflux gastritis, weight loss, and metabolic consequences. Also, there is increased morbidity and mortality risk with gastric resection as compared with HSV alone or vagotomy with pyloroplasty. Antrectomy with vagotomy may be most beneficial for patients with complicated PUD (e.g., patients with bleeding, obstruction, nonhealing, and/or recurrent ulcers).
Antrectomy typically leaves enough of a gastric remnant to allow for maintenance of adequate nutrition, but a bowel anastomosis is necessary. Antrectomy with Billroth I reconstruction (gastroduodenostomy) is the most physiologic anastomosis because it restores normal bowel continuity. Alternatively, a Billroth II reconstruction (gastrojejunostomy) may be performed. A loop of jejunum is brought up to the greater curvature side of the gastric remnant. The duodenal stump must be closed, and this may be technically challenging, especially if the duodenum is diseased.
Surgical Procedures for Gastric Resection
Partial gastrectomy may consist of resection of the antrum, distal two thirds of the stomach, distal 60% of the stomach, or high subtotal gastrectomy, depending on the type of disease (e.g., ulcer, cancer) and its location. Following gastric surgery, GI tract continuity requires an anastomosis and distal partial gastrectomy, and the distal partial gastrectomy procedure is named according to the type of anastomosis between the gastric remnant and small intestine, regardless of the extent of gastric resection. Surgical procedures to restore continuity following distal gastric resection include Billroth I and II procedures and Roux-en-Y gastrojejunostomy.
Following antrectomy for peptic ulcer disease, GI continuity is reestablished with a Billroth I gastroduodenostomy or Billroth II gastrojejunostomy. An additional choice for the gastrojejunostomy may be a Roux-en-Y gastrojejunostomy. However, when antrectomy is performed for ulcer disease, there is typically a large gastric remnant (60% to 70% of the stomach); Roux-en-Y gastrojejunostomy should be avoided in this setting. In the presence of a large gastric remnant, Roux-en-Y construction will predispose to gastric stasis and marginal ulcers. Distal gastrectomy for gastric neoplasm typically results in a smaller gastric remnant, and Roux-en-Y gastrojejunostomy may be preferable to prevent biliopancreatic secretions and duodenal contents from entering the gastric remnant and esophagus.
Billroth I Procedure
The Billroth I procedure consists of antrectomy with a gastroduodenal anastomosis ( Fig. 35-1 ). The distal gastric remnant is anastomosed to the end of the proximal duodenum. GI tract continuity is restored with preservation of the duodenal passage. Constructing the gastroduodenal anastomosis to approximate the size of the pylorus helps delay gastric emptying and reduce problems with postgastrectomy dumping. Because of anastomotic requirements, gastroduodenostomy may only be performed following antrectomy. For more significant gastric resections, a gastrojejunostomy is necessary. Also, in patients with gastric outlet obstruction caused by PUD, gastrojejunostomy is preferable to gastroduodenostomy.


Billroth II Procedure
The classic Billroth II procedure is a distal gastrectomy with a loop-type gastrojejunostomy ( Fig. 35-2 ). The gastric remnant is anastomosed to the side of the proximal jejunum. An area of the jejunum approximately 12 to 15 cm distal to the ligament of Treitz (the first or second loop of jejunum) is typically chosen and is brought to the greater curvature of the gastric remnant. The anastomosis may involve the entire end or a portion of the gastric remnant; an anastomosis along the dependent greater curvature of the gastric remnant facilitates gastric emptying. This then creates a proximal or afferent loop consisting of the oversewn duodenal stump and the most proximal jejunum, which carry the biliopancreatic secretions. There is then a distal or efferent loop extending downstream, carrying gastric contents distally. The gastrojejunostomy may be anterior to the transverse colon (antecolic) or posterior to the transverse colon (retrocolic). A retrocolic configuration allows for a shorter afferent loop, which may result in fewer postoperative complications.


Roux-en-Y Gastrojejunostomy
With a Roux-en-Y gastrojejunostomy, the jejunum is transected, and the proximal end of the jejunum (the Roux limb) is anastomosed to the gastric remnant, usually end-to-side ( Fig. 35-3 ). The small bowel may be brought up anterior or posterior to the transverse colon (antecolic or retrocolic, respectively). When the small bowel is brought up posterior to the transverse colon, an opening is made in the transverse mesocolon, which must be repaired. For malignancy, an antecolic location may be preferable to minimize obstruction caused by tumor recurrence in the lesser sac. The end of the proximal jejunal segment is then anastomosed to the more distal jejunum at least 40 to 60 cm from the gastrojejunal anastomosis. This procedure diverts duodenal contents and biliopancreatic secretions away from the stomach and prevents bile reflux into the stomach, which may help minimize bile reflux gastritis. However, transection of the small bowel alters intestinal motility, and there may be impaired emptying of the Roux limb as a consequence. A Roux-en-Y gastrojejunostomy should ideally be performed in the setting of a small gastric remnant to minimize marginal ulcer disease and problems with gastric stasis.

Identification of Postsurgical Anatomy on Imaging Studies
The postoperative anatomy following gastroduodenostomy or gastrojejunostomy may be evaluated with UGI or CT. Procedures for distal gastrectomy should be readily identified on UGI. Along with foreshortening of the stomach caused by distal resection, there is absence of a normal pylorus in a gastroduodenostomy, typically with an end-to-end anastomosis (see Fig. 35-1 ). This may be difficult or impossible to identify on CT if the gastric remnant consists of most of the stomach, and surgical clips or sutures are not present. In the setting of a gastrojejunostomy, jejunal loops will be contiguous with the gastric remnant. On CT, a jejunal limb will be contiguous with the gastric remnant and can be followed through its antecolic or retrocolic course. Sutures or staples may be seen along the proximal duodenal stump. On UGI, the gastrojejunal anastomosis should appear widely patent (see Fig. 35-2 ). A portion of the cut end of the stomach may be oversewn or inverted to restrict the size of the stoma, which can produce deformity and plication defects around the margins of the anastomosis. Plication defects can be mistaken for leaks, ulcers, or recurrent tumor if not appreciated. Unlike a plication defect, there will be persistent contrast in a leak following passage of the bolus. The adjacent gastric folds will appear thickened and lobulated in the case of recurrent ulcer or tumor as compared with postoperative deformity. Comparison with a baseline postoperative UGI may help clarify the process. Alternatively, endoscopy may be necessary.
Complications After Gastric Resection
Metabolic Problems
Anemia may be a consequence of gastric resection. Iron deficiency is the most common cause, but vitamin B 12 or folate deficiency may also occur. Contributing factors may include malabsorption, inadequate oral intake, and chronic bleeding. Iron deficiency anemia may be related to rapid passage through the proximal jejunum, with resultant decreased iron absorption. Also, decreased acid and pepsin may limit the conversion of organic iron to inorganic iron (absorbable by the GI tract). Loss of intrinsic factor production in the stomach can lead to vitamin B 12 deficiency.
Gastric surgery may also disturb calcium and vitamin D metabolism, resulting in metabolic bone disease. Calcium is absorbed in the duodenum, which is bypassed in a gastrojejunostomy. Also, fat malabsorption can occur because of bacterial overgrowth, insufficient mixing of food with digestive enzymes, or afferent loop syndrome. This can result in decreased absorption of fat-soluble vitamin D.
Postoperative Leak
Anastomotic leak or breakdown of a suture line can occur at any anastomosis in up to 5% of patients. Following a Billroth I procedure, leaks may occur at the gastroduodenal anastomosis. With a Billroth II, leaks may occur at the gastrojejunostomy or from the oversewn duodenal stump. With a Roux-en-Y reconstruction, leakage may also occur at these sites or, rarely, at the jejunojejunal anastomosis.
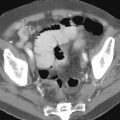
Water-soluble contrast should be used on UGIs if leakage is a concern. Extravasation of contrast material may be seen, with contrast opacifying a track or collection or coursing freely in the peritoneal cavity ( Fig. 35-4 ). A fistula may be seen with contrast opacifying another structure. Any postoperative drain should be carefully evaluated because opacification of a drain may be the only manifestation of a leak on UGI. CT may be necessary to determine the extent and location of collections and abscesses more definitively and to assess for leakage from the oversewn duodenum ( Fig. 35-5 ). Dehiscence of the duodenal stump suture line may be difficult to diagnose with imaging studies and can be a serious complication, resulting in leakage of bile and pancreatic sections into the peritoneal cavity. The duodenal stump may be difficult to opacify in retrograde fashion (anteperistaltic) on UGI. As a result, a fluoroscopic study that does not demonstrate an anastomotic leak following gastrojejunostomy does not exclude the presence of leakage, particularly from the oversewn duodenal stump. CT may reveal complex fluid or gas collections in the vicinity of the duodenal stump (see Fig. 35-5 ), in the subhepatic or peripancreatic space, or findings of peritonitis.


Gastric Stasis
Stasis in a gastric remnant can result in postprandial bloating, vomiting, abdominal pain, and weight loss. Gastric stasis occurs without evidence of mechanical intestinal obstruction or anastomotic narrowing in up to 25% of patients, depending on the type of reconstruction that was performed. Ineffective gastric emptying, impaired bowel motility, and alkaline reflux gastritis can contribute to gastric stasis. UGI should reveal a patent anastomosis without obstruction. Nuclear medicine studies may be of benefit to assess gastric emptying. Severe gastric stasis may require surgical revision with repositioning of the anastomosis or total gastrectomy.
Roux Syndrome
Roux syndrome, or Roux stasis syndrome, may occur following distal gastrectomy with a Roux-en-Y gastrojejunostomy. With this syndrome, patients have an atonic stomach with delayed emptying of the gastric remnant and Roux limb without evidence of obstruction. This represents a motility abnormality rather than a mechanical problem. Patients may present with vomiting, epigastric pain, and weight loss.
On UGI, there is dilation of the gastric remnant with delayed emptying. The efferent limb is typically dilated, without evidence of mechanical obstruction. It is important to assess the anastomosis to make sure that an anastomotic stricture is not a contributing factor. A gastric emptying nuclear medicine scan may also show delayed solid and/or liquid emptying. GI motility testing shows abnormal motility in the Roux limb, with propulsive activity toward the stomach rather than away from the stomach. Promotility medications may be beneficial.
Obstruction
Gastric outlet or small bowel obstruction can be an early or late postoperative complication following ulcer surgery. Obstruction may be caused by postoperative edema or hematoma in the early postoperative course. In contrast, late postoperative obstruction can be caused by anastomotic strictures, with or without stomal ulcers, adhesive disease, or internal hernias. UGI may reveal an anastomotic stricture with gastric outlet obstruction ( Fig. 35-6 ). The patient should be positioned at fluoroscopy so that the anastomosis is viewed in profile, without overlapping structures, to optimally assess anastomotic width and height. Endoscopic treatment and balloon dilation of strictures may be beneficial in some patients.

Bezoar Formation
A bezoar is a potential complication of subtotal gastrectomy. A bezoar may form in a gastric remnant as a result of decreased peristalsis and absence of gastric acid, especially when a vagotomy has been performed. Retained ingested material can coalesce to become a large masslike conglomerate. On UGI and CT, this appears as a large mottled mass within the gastric remnant. Contrast material and gas trapped within the interstices of the mass may help establish the correct diagnosis ( Fig. 35-7 ). Also, bezoars are usually mobile, allowing for distinction from a gastric tumor. A bezoar can lead to gastric outlet obstruction and occasionally small bowel obstruction if the bezoar passes into the jejunum. Bezoars may be successfully treated endoscopically, but surgical intervention may be necessary, especially with an associated obstruction.

Dumping Syndrome
Dumping is a phenomenon caused by destruction or bypass of the pyloric sphincter (as with pyloroplasty or antrectomy) with rapid gastric emptying; however, other factors likely play a role. Patients with early dumping syndrome experience vasomotor and cardiovascular symptoms, including tachycardia, dizziness, diaphoresis, weakness, nausea, abdominal cramping, diarrhea, and the need to lie down after eating. Postprandial symptoms may be severe and disabling. High-carbohydrate food may precipitate the worst attacks. A rapid influx of sugars into the proximal jejunum may cause a strong osmotic effect, drawing fluid into the bowel.
Crampy abdominal pain and distention are not uncommon, and diarrhea often follows. Early dumping occurs 15 to 20 minutes after a meal in 5% to 50% of patients, depending on the specific operation. Clinically significant dumping occurs in 5% to 10% of patients after pyloroplasty or distal gastrectomy. Because of the abdominal pain associated with eating, patients tend to lose weight and become malnourished.
UGI in early dumping syndrome may reveal very rapid transit time with rapid opacification of the colon. Conservative therapy for dumping syndrome consists of dietary management; most patients improve with dietary changes over time. A somatostatin analogue may be beneficial. Surgical management may be necessary in a small percentage of patients, but the results of surgery for dumping symptoms are variable and unpredictable.
Late dumping, also called postprandial (reactive) hypoglycemia, occurs 2 to 4 hours after a meal and may have similar vasomotor symptoms, but does not typically have the associated GI symptoms. This can be alleviated by the administration of carbohydrates.
Stomal and Anastomotic Ulceration
Recurrent ulcers following gastric surgery for PUD occur at or near the anastomosis, usually on the duodenal or jejunal side of the anastomosis for a Billroth I or II procedure, respectively. In a Billroth II, the efferent loop is more commonly involved than the afferent loop. These ulcers are referred to as stomal, marginal, or postanastomotic ulcers; they occur primarily following inadequate gastric resection with a retained segment of antrum or because of a hypersecretory state or gastrinoma. In the setting of a Roux-en-Y gastrojejunostomy for PUD, a large gastric remnant predisposes to marginal ulcers.
UGI may reveal an ulcer crater with surrounding edema ( Fig. 35-8 ). Stomal ulcers may be difficult to diagnose on UGI studies because of opacification of overlapping structures, and it may be difficult to distinguish barium trapping in distorted folds and plication defects from an ulcer. A baseline postoperative comparison study may be most beneficial to make this distinction. Secondary signs of ulcers may include narrowing at the anastomosis with thickened, edematous, and masslike folds. UGI findings may be less reliable compared with endoscopy, but contrast studies are increasingly necessary when endoscopy is limited or difficult. If recurrent ulcers fail to respond to medical management, total gastrectomy may be necessary.

Jejunogastric Intussusception
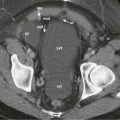
Intussusception may occur in the vicinity of any anastomosis. Jejunogastric intussusception is a rare complication following gastrojejunostomy, with a reported incidence of 0.1%; the efferent loop intussuscepts into the stomach more often than the afferent loop. Prolapse may occur in an antegrade or retrograde direction. Retrograde migration of the jejunal segment into the gastric remnant can be acute, chronic, or intermittent and can result in partial or complete gastric outlet obstruction. On CT, the proximal jejunum, along with the adjacent mesenteric fat and vessels, can be seen within the lumen of the gastric remnant. On UGI, contrast can be seen outlining the jejunal folds within the gastric remnant, producing a coil spring appearance ( Fig. 35-9 ). A gastrojejunal intussusception may be manifested by narrowing of the distal gastric remnant with a coil spring filling defect in the adjacent proximal jejunum.

Afferent Loop Syndrome
Following gastrojejunostomy, the afferent loop (duodenal stump containing biliopancreatic secretions) may become dilated, which can lead to abdominal pain, nausea, vomiting, postprandial fullness, and, rarely, obstructive jaundice. This has been called afferent loop syndrome ; it occurs in less than 1% of patients. Afferent loop syndrome may be caused by blockage of this limb and may occur with adhesions, kinking or fibrosis near the anastomosis, internal hernias, or volvulus. Partial obstruction of the afferent loop results in luminal dilation with accumulation of biliary, pancreatic, and duodenal secretions. Acute complete obstruction may also occur. Afferent loop syndrome can also be due to preferential flow of ingested contents into the afferent limb (retrograde) rather than into the efferent limb.
Diagnosis of afferent loop syndrome caused by obstruction may be best made on CT. CT reveals a dilated, fluid-filled afferent loop ( Fig. 35-10 ) and may also reveal the cause of obstruction. On UGI, the obstructed afferent loop may not be opacified. The diagnosis may be inferred by the absence of opacification of the afferent limb and mass effect on the opacified efferent limb by the fluid-filled, unopacified afferent segment. However, lack of opacification of the afferent limb occurs in up to 20% of all patients following gastrojejunostomy, so lack of opacification by itself is not a reliable indicator of afferent loop syndrome. Afferent loop syndrome can also be due to preferential flow of ingested contents into a dilated afferent limb rather than the efferent limb, and this can be readily identified with UGI (see Fig. 35-10C ). In afferent loop syndrome, hepatobiliary scintigraphy reveals retention in the dilated biliopancreatic segment.

Bile Reflux Gastritis, Chronic Remnant Gastritis
A common complication following gastric surgery is chronic gastritis. Bile reflux gastritis occurs in 5% to 15% of patients following partial gastrectomy. Without an intact pylorus, chronic reflux of bile and pancreatic secretions into the gastric remnant can result in inflammatory change in the residual stomach. Following resection of the pylorus, most patients have bile in the stomach, with some degree of inflammation on endoscopic examination, and therefore many patients remain asymptomatic in the setting of bile reflux. However, a subset of patients will have bile reflux gastritis and present with nausea, bilious vomiting, and epigastric pain, possibly with excessive enterogastric reflux. There does not appear to be a strong correlation between the degree of inflammation and the degree of symptoms. The onset of pain is not associated with meals. The differential diagnosis may include afferent loop syndrome, obstruction, and gastric stasis, and UGI may help establish the diagnosis. UGI may reveal thickened gastric folds without associated obstruction ( Fig. 35-11 ). Ulcers may or may not be present in the perianastomotic region of the gastric remnant.

Conversion to Roux-en-Y gastrojejunostomy with at least 45 cm of isoperistaltic jejunal loop between the gastric remnant and duodenum may help alleviate bile reflux gastritis by diverting bile and pancreatic secretions away from the gastric remnant.
Neoplasms After Surgery for Benign Ulcer Disease
Following partial gastrectomy for benign disorders, especially PUD, there is an increased risk of gastric remnant neoplasms. This occurs in the late postoperative course, with a 15- to 20-year latent period, at which point the relative risk increases twofold to fourfold. Malignant tumors most likely develop as a result of gradual progression from normal mucosa to intestinal metaplasia to dysplasia, and, subsequently, to cancer over time, presumably as a consequence of prolonged achlorhydria and chronic enterogastric reflux of bile and pancreatic secretions. Truncal vagotomy reduces gastric acid and may contribute to this process. Stump tumors involve the distal gastric remnant at or near the anastomosis. On UGI, stump tumors appear as infiltrating, plaquelike, polypoid, or ulcerative lesions in the gastric remnant near the anastomosis.
Surgical Considerations for Gastric and Duodenal Neoplasms
Gastric and duodenal neoplasms are discussed in detail elsewhere in this textbook. Neoplasms of the stomach and duodenum require resection, with the extent and location of resection dependent on the benign or malignant nature of the lesion, lesion location, extent of disease, and underlying physiologic status of the patient.
Surgery for Gastric Masses
The goal of surgical treatment for gastric tumor is to remove the grossly visible tumor completely and obtain histologically free surgical margins. The extent of the procedure depends on the degree of tumor infiltration in and through the gastric wall, extension into adjacent organs, and lymph node involvement. Upper endoscopy and endoscopic ultrasound (EUS) can be useful to determine the location and extent of disease spread within the gastric wall. It is essential to properly stage any patient who is being considered for subtotal gastrectomy for cancer prior to operative intervention. For example, when a total gastrectomy is planned, the proximal extent of disease should be determined preoperatively to determine whether a distal esophagectomy is also necessary.
There are several different surgical procedures that may be performed to treat gastric masses. The size and location of the tumor, in conjunction with the surgeon’s experience and preference, help determine the exact surgical procedure performed. Malignant gastric lesions usually require distal or total gastrectomy. Surgical procedures for gastric cancer may include a Billroth II, Roux-en-Y gastrojejunostomy, Roux-en-Y esophagojejunostomy, or primary esophagogastric anastomosis. Omentectomy and/or splenectomy may be performed simultaneously, if necessary.
The location of the primary tumor determines the type of resection performed. For a lesion located in the mid to distal stomach, particularly along the greater curvature, subtotal gastric resection may be performed, ideally with margins of 5 to 6 cm around the lesion. A gastroenterostomy is performed to restore continuity of the GI tract. The need for wide margins usually requires a gastrojejunostomy rather than gastroduodenostomy, and a Billroth I is not typically performed for malignant disease. Gastrojejunostomy may be a loop-type or Roux-en-Y configuration.
Larger and more proximal tumors may require total gastrectomy with an esophagojejunal anastomosis, typically a Roux-en-Y esophagojejunostomy. The distal esophagus may also be resected with a more cephalad esophagojejunal anastomosis. For lesions in the proximal stomach, particularly within 5cm of the gastroesophageal junction, proximal gastric resection may be possible with a primary esophagogastric anastomosis. However, proximal subtotal gastrectomy with esophagogastric anastomosis is not commonly performed because of problems with gastroesophageal reflux, and the anastomosis between the esophagus and distal stomach may be less secure, resulting in an increased risk of anastomotic leaks. Total gastrectomy with esophagojejunostomy may be preferable. However, anastomotic leaks are of particular concern following esophagojejunostomy, occurring in up to 12% of patients and with a mortality of approximately 33% ( Fig. 35-12 ).