The imaging of treated gliomas is complicated by a variety of treatment related effects, which can falsely simulate disease improvement or progression. Distinguishing between disease progression and treatment effects is difficult with standard MR imaging pulse sequences and added specificity can be gained by the addition of advanced imaging techniques.
Key points
- •
Clinical information is key to the correct interpretation of changes in imaging findings in treated gliomas.
- •
Subacute ischemia, blood–brain barrier breakdown related to recent surgery, pseudoprogression, and delayed radiation necrosis can cause increased or new foci of enhancement that do not reflect true progression of disease.
- •
Both antiangiogenic therapy and increases in steroid dosage can decrease tumor enhancement without affecting the underlying disease burden.
- •
Perfusion, spectroscopy, and PET can add specificity in differentiating treatment effects from true disease progression.
Introduction
Gliomas are the most common primary intracranial malignant neoplasm in adults. Among these, glioblastoma exhibits the greatest incidence, and simultaneously carries the highest grade and a dismal prognosis. Lower grade glial neoplasms can range from nonaggressive lesions, amenable to curative treatment such as ganglioglioma, to infiltrative neoplasms with a high rate of transformation to higher grade disease. The World Health Organization classification segregates glial neoplasms into different grades based on resectability and proliferative potential. The primary radiologic challenges are found in imaging gliomas of grade II or higher; the most commonly encountered such tumors include diffuse astrocytomas, oligodendrogliomas, anaplastic astrocytomas, and glioblastomas. These challenges are exacerbated in the posttreatment setting, particularly when imaging high-grade gliomas (World Health Organization grades III and IV lesions).
Complete surgical resection of diffuse gliomas is often compromised by the infiltrative nature of these tumors and the presence of tumor cells that lie beyond the tumor margin delineated by conventional imaging. The current treatment paradigm for high-grade glial neoplasms begins with maximal safe resection of the enhancing portion of the tumor. If the entirety of the enhancing component can be resected safely, this is termed a gross total resection. This is followed by adjuvant therapy, the composition of which depends on the tumor’s histology and cytogenetics. For glioblastoma, the current treatment paradigm status after primary resection is treatment with involved field radiation therapy and temozolomide, with recent possible consideration for the additional implementation of an alternating electric fields/tumor treating fields device. Patients with primary treatment failure or recurrence may receive a variety of therapies; perhaps the most pertinent of these to the practicing radiologist is anti-vascular endothelial growth factor (VEGF) therapy, commonly undertaken with bevacizumab (an anti–VEGF-A antibody with the trade name Avastin). In this work, we explore a variety of current imaging approaches that attempt to distinguish posttreatment areas of true tumor progression from their common mimics.
Introduction
Gliomas are the most common primary intracranial malignant neoplasm in adults. Among these, glioblastoma exhibits the greatest incidence, and simultaneously carries the highest grade and a dismal prognosis. Lower grade glial neoplasms can range from nonaggressive lesions, amenable to curative treatment such as ganglioglioma, to infiltrative neoplasms with a high rate of transformation to higher grade disease. The World Health Organization classification segregates glial neoplasms into different grades based on resectability and proliferative potential. The primary radiologic challenges are found in imaging gliomas of grade II or higher; the most commonly encountered such tumors include diffuse astrocytomas, oligodendrogliomas, anaplastic astrocytomas, and glioblastomas. These challenges are exacerbated in the posttreatment setting, particularly when imaging high-grade gliomas (World Health Organization grades III and IV lesions).
Complete surgical resection of diffuse gliomas is often compromised by the infiltrative nature of these tumors and the presence of tumor cells that lie beyond the tumor margin delineated by conventional imaging. The current treatment paradigm for high-grade glial neoplasms begins with maximal safe resection of the enhancing portion of the tumor. If the entirety of the enhancing component can be resected safely, this is termed a gross total resection. This is followed by adjuvant therapy, the composition of which depends on the tumor’s histology and cytogenetics. For glioblastoma, the current treatment paradigm status after primary resection is treatment with involved field radiation therapy and temozolomide, with recent possible consideration for the additional implementation of an alternating electric fields/tumor treating fields device. Patients with primary treatment failure or recurrence may receive a variety of therapies; perhaps the most pertinent of these to the practicing radiologist is anti-vascular endothelial growth factor (VEGF) therapy, commonly undertaken with bevacizumab (an anti–VEGF-A antibody with the trade name Avastin). In this work, we explore a variety of current imaging approaches that attempt to distinguish posttreatment areas of true tumor progression from their common mimics.
Tumor biology
The typical high-grade glioma demonstrates 3 radiologic “zones.” The first zone is defined by the enhancing core of the tumor, in which neoangiogenesis can result in a variety of aberrant vessel subtypes, ultimately leading to breakdown of the blood–brain barrier and leakage of radiologic contrast agent. This zone of neovascular proliferation is important because it is both a cardinal feature of high-grade glioma as well as a potential target for antiangiogenic therapy, discussed in greater detail elsewhere in this article. The second zone is the perilesional area of T2/fluid attenuation inversion recovery (FLAIR) signal abnormality surrounding the core of the lesion, which comprises a mix of nonenhancing infiltrative tumor and vasogenic edema, sometimes referred to as infiltrative edema . The third zone is the surrounding, normal-appearing brain parenchyma that harbors microscopic tumor at levels that are not currently detectable on conventional, routine 3T anatomic MR pulse sequences. Although promising research is being undertaken currently to better define the extent of nonenhancing tumor burden using advanced imaging techniques such as quantitative magnetization transfer, the full extent of the tumor is currently defined poorly in clinical practice. This is in part owing to the microscopic extensions of perilesional tumor that provide a mechanism for the apparent “skip lesions” identified when new sites of disease appear distant to previously perceived margins of the tumor, and help to explain the mechanism behind cases presenting with multifocal disease. Ultimately, it is the diffuse infiltrative nature of these lesions coupled with their relative resistance to chemoradiation that supports the observation that diffuse gliomas are largely, presently incurable.
In this context, tumor genetics is becoming increasingly relevant to the treatment of neoplasms. The recently released 2016 revision to the 4th edition of the World Health Organization (WHO) criteria for tumors of the Central Nervous System reflects an increasing emphasis on tumor genetics as they pertain to tumor behavior and therapeutic response. A detailed discussion of tumor genetics is beyond the scope of this article, but the radiologist should be made aware of a few of the more relevant genetic markers for adult gliomas. The new WHO criteria place an emphasis on mutations of isocitrate dehygrogenase (IDH) and the codeletion of 1p and 19q loci as important determinants of tumor behavior. IDH mutation, most commonly IDH-1, is positively correlated with survival versus the wild type gene product. 1p 19q codeletion is also a mutant variant which demonstrates an improved survival; in addition, mutation of IDH-1 and codeletion of 1p 19q is now recognized as the genetic signature of an oligodendroglioma. The gene, O6 methylguanine—DNA methyltransferase (MGMT), encodes an enzyme involved in DNA repair and has important therapeutic implications that can potentially impact radiological interpretation. Specifically, when the promoter of this gene is hypermethylated, its activity is downregulated and gliomas and other tumors are more susceptible to DNA damage from alkylating agents such as temozolomide. Familiarity with these genetic marker subtypes and their effect on the behavior of high-grade gliomas is important for proper interpretation of imaging studies.
Imaging time frame
Imaging is usually performed within the first 24 to 48 hours after maximal safe resection but should be undertaken within the first 72 hours to establish a new baseline while minimizing the confounding effects of postoperative changes. The authors’ institution currently performs follow-up imaging 4 weeks after the completion of chemoradiation to allow for the reduction of acute radiation- and chemotherapy-related changes before evaluation for a possible therapeutic response. However, it has been advocated that subsequent imaging may be delayed up to 12 weeks in nonenhancing tumors (eg, low-grade gliomas) to allow for complete resolution of postoperative edema and improved assessment of the true extend of tumor resection.
Challenges with imaging
In the untreated patient, the enhancing component of a tumor can represent a surrogate of high-grade disease with microvascular proliferation. However, once the patient has undergone treatment, a variety of therapeutic interventions can cloud this picture, causing increases or decreases in the amount of apparent contrast enhancement without a significant effect on the actual burden of high-grade disease. Because the amount of enhancing disease is a major criterion for therapeutic response in the currently implemented oncologic imaging criteria, the Response Assessment in Neuro-Oncology (RANO) criteria, it is important to consider that changes in enhancement may not necessarily reflect changes in tumor burden.
Mimics of Progression (Increased Enhancement)
Subacute infarction and postoperative blood–brain barrier disruption
In the postoperative state, ischemia surrounding the resection cavity can demonstrate avid enhancement in the subacute phase, often mimicking residual, enhancing disease and result in an erroneous diagnosis of residual high-grade tumor. To prevent this error, postoperative imaging is typically performed within the first 24 to 48 hours after tumor resection to establish a new baseline, while minimizing the confounding effects of evolving postoperative changes.
Pseudoprogression
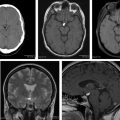
At the authors’ institution, posttreatment imaging is generally performed 4 weeks after the completion of chemoradiation. In this early posttreatment stage, an increase in the contrast-enhancing portion of the tumor can represent 2 entities: (1) true early progression of disease or (2) an entity known as pseudoprogression. Pseudoprogression is a complex subacute treatment-related response in which abnormally enhancing tissue demonstrates transient increased vascular permeability, edema, and necrosis, with a reported incidence of 20% to 30%. The cardinal feature of this entity that distinguishes it from true early progression on standard sequences is that it stabilizes and subsequently improves (and/or subsides) without any further treatment. Pseudoprogression is most commonly seen in the first 3 months after the conclusion of therapy, but can be seen up to 6 months after treatment ( Fig. 1 ). It is correlated positively with improved survival, and hypermethylation (inactivation) of the MGMT promoter gene is associated with an increased incidence of pseudoprogression.
In the face of increasing enhancement and in the appropriate time frame, it is the opinion of the authors that findings identified with advanced imaging techniques can often further differentiate a diagnosis of true early progression from that of pseudoprogression (discussed elsewhere in this paper), but follow-up imaging has historically been the preferred, noninvasive method to differentiate these respective diagnoses with clinical certainty, as demonstrated in Fig. 2 .
Delayed radiation necrosis
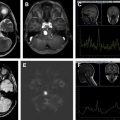
This entity is most commonly seen 9 to 12 months after treatment but can be seen years after initial therapy. Radiation necrosis presents as a focus or curvilinear region of new contrast enhancement, often ringlike, within the field of prior radiation treatment. As with pseudoprogression, differentiating radiation necrosis from recurrent tumor is often difficult using standard, conventional anatomic sequences, particularly if the enhancement is seen within the high dose portion of the radiation field. Perfusion imaging can be helpful ( Fig. 3 ) but the disparity in published results, using a variety of “perfusion” techniques, each with a myriad imaging parameters and postprocessing methods, has resulted in significant heterogeneity in the literature; these observations make it difficult to draw concrete conclusions and accept associated discrete cutoff values that might otherwise be used to rule in or out the diagnosis of radiation necrosis.
Mimics of Improvement (Decreased Enhancement)
Pseudoresponse
Pseudoresponse is the radiologic term used to describe a decrease in the size of the enhancing component of a tumor in the setting of antiangiogenic therapy. In adult high-grade glioma therapy, the antiangiogenic medication of choice is currently bevacizumab. Bevacizumab is a monoclonal antibody against VEGF type A. It is used most commonly as a secondary therapy for glioblastoma progression after initial therapy has failed, although it is being investigated in other roles, including upfront, concomitant first-line therapy. When used as a secondary treatment, it can provide reported symptom improvements and often allows for reduction in simultaneous steroid dosage, but without a definite effect on prolonged overall survival. This medication is known to produce striking improvements in the imaging “appearance” of the tumor, with marked decrease in tumoral enhancement.
Effects on perfusion are more complex depending on which technique and parameter are being investigated. Overall, cerebral blood volume (CBV), cerebral blood flow (CBF), and the transfer constant ( K trans , a measure of vascular permeability), all tend to decrease in response to initial antiangiogenic therapy, as illustrated with CBF in Figs. 4 and 5 ; this latter case is also illustrative of vessel co-option, in which there is a shift in tumor physiology such that tumors initially reliant on neo-angiogenesis may shift to a more infiltrative nature, harnessing their blood supply by growing along perivascular spaces. As such, the effects of antiangiogenic medications on enhancement and perfusion parameters are owing to changes in the tumor vasculature, including decreased vascular permeability, decreased patency of preexisting blood vessels, and decreased vascular proliferation. Although these changes can result in the improvement of reported patient symptoms, it is important not to interpret the imaging changes as an antitumoral effect, but rather as a change in tumor vascularity and its associated imaging appearance. Other imaging biomarkers become more important in the setting of antiangiogenic therapy. The use of apparent diffusion coefficient (ADC) values in the setting of antiangiogenic therapy has been extensively studied.
Radiologic interpretations based on routine pulse sequences
Response Assessment in Neuro-Oncology Criteria
The RANO criteria are the imaging guidelines used to assess disease progression, stability, and therapeutic response in current clinical trials and routine clinical care. Although rudimentary, these criteria should guide interpretations performed using standard pulse sequences acquired in postoperative brain tumors. The RANO imaging criteria are split into 2 distinct blocks of time, the first 12 weeks after completion of chemoradiotherapy and the time period after the first 12 weeks; this is done in recognition of the current difficulty that conventional imaging has in identifying true early progression in the immediate posttherapy period. Using these criteria, disease progression cannot be determined by conventional imaging alone in the first 12 weeks after completion of chemoradiation unless there is new enhancement outside the high-dose region of the radiation port. After 12 weeks, the guidelines become more complex, but take into account information that may not be easily obtainable by the radiologist, including clinical status and changes in corticosteroid doses. However, the 2 main imaging surrogates of disease recognized by the RANO criteria are the enhancing component and the T2/FLAIR component of disease. Table 1 summarizes these important imaging criteria.
| Criterion | Complete Response | Partial Response | Stable Disease | Progressive Disease a |
|---|---|---|---|---|
| Enhancement | None | ≥50% decrease | <50% decrease but >25% increase | ≥25% increase |
| T2/FLAIR | Stable to decreased | Stable to decreased | Stable to decreased | Increased |
| New lesion | No | No | No | Yes |
| Corticosteroid dose | None | Stable to decreased | Stable to decreased | N/A |
| Clinical status | Stable or improved | Stable or improved | Stable or improved | Declining |
| Requirement for response | All of the above | All of the above | All of the above | Any |
a In the first 12 weeks, only histology or new enhancement outside the radiation field can indicate progression.
Clinical data to gather before beginning interpretation
There are several key pieces of clinical data that should shape the interpretation of the imaging findings in a posttreatment glioma, summarized in Table 2 . The most crucial piece of information is the date when radiotherapy was completed; without this piece of data, interpretation of the significance of an increase in enhancement is problematic. It is also imperative to establish if the patient is currently on antiangiogenic therapy or was receiving the medication previously, at the time a prior comparison examination was acquired, owing to the drastic effects of this class of medications on the enhancement pattern of the tumor. Knowledge of the patient’s current steroid dose and any changes in dosage from the most recent comparison can also help interpretation of changes in T2/FLAIR signal and enhancement. Although often technically challenging from an information technology perspective, it can also be extremely useful to have the radiation treatment field available in PACS (picture archiving and communication system) to determine whether new enhancing foci are within the high dose zone of the radiation treatment field. Finally, knowledge of the MGMT methylation status of the evaluated tumor is important from an imaging perspective, because MGMT hypermethylated (deactivated) tumors are more likely to undergo pseudoprogression and attempting to differentiate pseudoprogression from true early progression using dynamic susceptibility contrast (DSC)-based relative CBV (rCBV) may be less useful in MGMT hypermethylated tumors.
| Clinical Parameter | Imaging Effect(s) |
|---|---|
| Date of completion of chemoradiotherapy | Alters interpretation of areas of increasing or new enhancement in the radiation field: pseudoprogression in the first 6 mo and delayed radiation necrosis from 9 mo on. |
| Antiangiogenic therapy | Alters interpretations of areas of increasing or decreasing enhancement. |
| Alters what the radiologist should monitor for disease progression: restricted diffusion becomes much more important and T2/FLAIR becomes more important than enhancement. | |
| Radiation field | Helps to decide if a new focus of enhancement represents multifocal disease or could possibly be owing to radiation necrosis. |
| MGMT status of tumor | MGMT hypermethylated tumors more often undergo pseudoprogression. |
| Pseudoprogression in MGMT hypermethylated tumors have a better outcome. | |
| rCBV may be less useful to differentiate pseudoprogression from true early progression in MGMT hypermethylated tumors. | |
| Change in steroid dose from prior imaging study | Changes in steroid doses can affect the size of the T2/FLAIR component of the lesion as well as the size of the enhancing component of disease. |
Enhancement
Any new foci of enhancement beyond the high-dose radiation zone are concerning for spread of high-grade tumor regardless of the time frame. Using the RANO criteria, an increase by 25% or more in the enhancing component of a tumor (including new foci within the radiation field) after the first 12 weeks after completion of chemoradiation is seen as disease progression. In practice, however, new areas of enhancement within the radiation field must be interpreted with caution as these areas could also represent pseudoprogression/radiation necrosis. Advanced imaging techniques can help to differentiate these entities but ultimately the determination becomes clinical. As with any other area of suspected enhancement, it is crucial to check for T1 signal on the unenhanced sequence because posttreatment changes can result in blood products and mineralization with intrinsic T1 hyperintensity that can easily simulate enhancement on the postcontrast sequence.
T2/Fluid Attenuation Inversion Recovery Imaging
Unlike contrast enhancement, the common clinical interpretation of T2/FLAIR imaging changes currently invokes more of a qualitative assessment, although quantitative volumetry has been successfully performed. As discussed, the T2/FLAIR changes surrounding the enhancing component of a tumor typically represent a combination of vasogenic edema and infiltrative tumor without discrete microvascular proliferation that is typical of blood–brain barrier breakdown and associated tumoral enhancement. This is further complicated in the posttreatment setting by treatment-related changes including tissue injury and concomitant laminar necrosis. Differentiating between changes in edema, gliosis, and other treatment-related effects is difficult, but signs that suggest tumor involvement include a bulky, masslike component and new signal alteration that extends into the cortex (see Fig. 5 ). A variety of advanced imaging techniques have been investigated that attempt to differentiate treatment related changes from definite tumor infiltration and are discussed elsewhere in this paper.
Diffusion Signal
A basic diffusion-weighted sequence is commonly obtained in most MR imaging protocols and should be obtained as part of a basic glioma follow-up. The usefulness of the ADC map has been investigated in a variety of settings, including estimating the grade of a primary brain neoplasm, differentiating between treatment changes and true progression, and evaluating for disease progression in the setting of antiangiogenic therapy. In these studies, high-grade tumor was shown to have lower ADC values than treatment-related effects when compared with contralateral white matter owing to the hypercellularity of high-grade tumor. The ADC, then, can be useful in suggesting that nonenhancing T2/FLAIR changes are tumor related rather than treatment related and are also useful in monitoring a patient in the setting of antiangiogenic therapy when the degree of enhancement will no longer reliably identify active, high-grade tumor. More advanced analyses of ADC values have also been performed. A lower mean ADC was found to correlate to decreased survival when segmented using a double Gaussian mixture model. In the setting of antiangiogenic therapies, a thresholded voxel-based analysis of the ADC has been shown to predict outcomes. Serial ADC maps have also been used to generate maps of cell invasion, motility, and proliferation level estimate. In these studies, cell invasion, motility, and proliferation level estimate maps were able to predict areas of tumor recurrence and predict progression free-survival. However, the significance of diffusion-restricting tissue may differ in the setting of antiangiogenic therapy, as illustrated in a study by Mong and colleagues, in which increased overall survival was observed in patients treated with bevacizumab in whom persistent diffusion-restricting tissue was identified. This observation lead the authors to postulate that the diffusion restriction may represent hypoxic tissue rather than hypercellular, aggressive tumor.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree