Chest computed tomography (CT) is the modality of choice for mediastinal imaging. The high-resolution images provided by multi-detector CT result in routine visualization of normal anatomic structures, which can be confused with pathology. In addition, many mediastinal abnormalities are discovered incidentally, with a routine chest CT protocol which may be insufficient for definite diagnosis. Awareness of the spectrum of potential pitfalls of mediastinal imaging, artifacts related to flow, motion, and solutions to mitigate these problematic issues is important in accurate interpretation. The purpose of this review is to highlight and discuss potential pitfalls in the imaging of the mediastinum.
Key points
- •
Advances in computed tomography technology allow visualization of normal anatomic structures and variants in greater detail, which can be misinterpreted as pathology.
- •
If a mediastinal mass is suspected to be cystic, MR imaging adds value by demonstrating its fluid content and is helpful to differentiate benign from malignant disease.
- •
An awareness of the spectrum of the potential pitfalls of mediastinal imaging and artifacts related to flow, motion, and cardiac pulsations is important for accurate interpretation.
- •
Flow artifacts can be resolved by obtaining a delayed venous scan.
- •
Artifacts owing to cardiac motion can be mitigated by using electrocardiographic gating.
Introduction
A computed tomography (CT) scan of the chest is the modality of choice for evaluating many suspected or known thoracic abnormalities involving the lungs and mediastinum. CT scans are widely available with a relatively quick acquisition time and allows for accurate and reproducible images of the mediastinum, its contents, and any associated abnormalities. Advances in multidetector CT technology, with improved temporal and spatial resolution, allow for better delineation of mediastinal structures. However, the high resolution of a CT scan also results in the routine visualization of normal anatomic structures and anatomic variants, which can be confused with pathology, as well as benign lesions that may mimic malignant neoplasms.
A CT scan may be performed for a variety of clinical scenarios, both in the acute and ambulatory settings. Ideally, the CT protocol is optimized and tailored to answer a specific clinical question or concern. However, many mediastinal abnormalities are discovered incidentally, and a CT scan of the chest performed in the routine fashion may be insufficient for the purposes of developing a focused differential diagnosis. Some artifacts induced by technique may simulate pathology, and additional evaluation with electrocardiographic (ECG)-gated cardiac CT scans, MR imaging, or a PET/CT scan with fluorodeoxyglucose (FDG) may add value. An awareness of the potential pitfalls in assessing the mediastinum on imaging studies and effective solutions to mitigate these problematic issues is necessary to ensure an accurate interpretation by radiologists. The purpose of this review is to highlight and discuss the potential pitfalls in the imaging of the mediastinum.
Cystic structures
Cystic or fluid-containing structures are commonly encountered in all compartments of the mediastinum, and may represent normal anatomy (such as fluid in the pericardial recesses), cysts (such as thymic or bronchogenic cyst), or cystic neoplasms (such as cystic thymoma). It is important to differentiate cystic neoplasms from benign lesions and normal anatomic structures to ensure appropriate intervention and avoid delays in treatment planning.
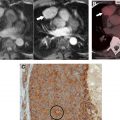
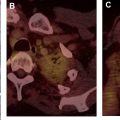
In the oncologic setting, one of the most commonly encountered mediastinal anatomic pitfalls relates to pericardial recesses mimicking lymphadenopathy. The pericardium is composed of an outer fibrous component and an inner serous component, which itself has an inner visceral layer, adherent to the heart and great vessels, and an outer parietal layer, which lines the fibrous pericardium. The pericardial space normally contains approximately 15 to 30 mL of fluid between the visceral and parietal layers of the serous pericardium. There are reflections of serous pericardium between the great vessels and at the base of the heart. Physiologic amounts of fluid in these pericardial reflections may vary between imaging studies obtained at different time points. Typical imaging features of fluid-filled pericardial sinuses and recesses include fluid attenuation, contiguity with other pericardial spaces, and no mass effect on adjacent structures. Multiplanar reformations are particularly helpful in demonstrating the relationship between the pericardial recesses and other mediastinal structures, as well as the contiguity with the rest of the pericardium. A distinctive beak-like appearance is seen as fluid in the pericardial recesses drapes over the neighboring mediastinal structures. Of the various pericardial recesses, the high-riding variant of the superior aortic recess extending cephalad into the right paratracheal region constitutes a potential pitfall in oncologic imaging because it can be confused with lymphadenopathy ( Fig. 1 ). Owing to its typical appearance, the use of other imaging modalities to confirm pericardial fluid is usually not necessary. However, when in doubt, MR imaging, with its superior contrast resolution, can show the fluid content with high signal intensity on T2-weighted images, low signal intensity on T1-weighted images, and the lack of enhancement after the administration of intravenous contrast. On CT scans, mediastinal lymphadenopathy manifests as soft tissue lesions with lobular margins that may exert a mass effect on the adjacent structures. Depending on the underlying etiology, enlarged lymph nodes may enhance after the administration of intravenous contrast. FDG PET/CT scans can also help to distinguish lymphadenopathy from the pericardial fluid, with the former demonstrating increased FDG uptake in the setting of metastatic disease or active infection or inflammation.

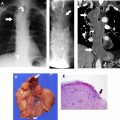
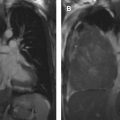
Mediastinal cysts are most commonly encountered in the prevascular compartment, typically within the fat anterior to or lateral to the pericardium. Although surgical series have suggested that cysts represent less than 5% of mediastinal masses, a recent multi-institutional study has shown that mediastinal cysts represent 24% of prevascular mediastinal masses. When a cystic lesion is identified in the prevascular mediastinum, the primary role of the radiologist is to differentiate between a benign cyst such as a thymic or pericardial cyst and a cystic thymoma. Thymic cysts are among most common benign thymic lesions and may be either congenital or acquired. Congenital thymic cysts are unilocular, contain clear fluid, and are found incidentally in asymptomatic patients during the first 2 decades of life. , Acquired thymic cysts may be multilocular and contain heterogeneous fluid indicating a high protein content owing to infection or hemorrhage. Conditions associated with thymic cysts include thymic neoplasms, radiation therapy, thoracotomy, and chest trauma. Unfortunately, thymic cysts often manifest with internal heterogeneous attenuation that cannot be differentiated from cystic neoplasms on CT imaging alone. A common pitfall in the evaluation of benign thymic cysts is misinterpretation as solid masses when their attenuation is higher than expected for fluid (higher than 20 Hounsfield units), which may then lead to unnecessary invasive tissue sampling or surgery. To address this issue, MR imaging can be performed to evaluate these lesions, because it is the optimal imaging modality for distinguishing cystic from solid lesions, identifying cystic and/or necrotic lesion components, characterizing cystic lesions as to the presence of septations and mural nodularity, and distinguishing normal or hyperplastic thymic tissue from neoplasia. Typical characteristics of a thymic cyst on MR imaging include a low signal intensity on T1-weighted images and a uniform high signal intensity on T2-weighted images. After the administration of intravenous contrast, thick, enhancing septations or mural nodularity should be absent ( Fig. 2 ). The presence of these findings should raise concern for a thymic malignancy. A high signal intensity on both T1- and T2-weighted images may represent a cystic lesion complicated by hemorrhage or infection. To avoid misinterpretation of a thymic malignancy as benign, it is important to pay close attention to the T1-weighted images after intravenous contrast administration, because enhancement is noted in the early dynamic phase in 90% of tumors, and the remaining 10% show enhancement on delayed T1-weighted images.
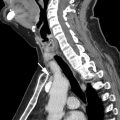
Tracheal diverticula are outpouchings from the tracheal wall that are lined with ciliated columnar epithelium. They are usually 5 to 25 mm in size. Although tracheal diverticula are connected to the airways by definition, the connecting stalk is frequently below the spatial resolution of a CT scan. These diverticula characteristically present as small air bubbles along the right posterolateral wall of the upper trachea (at the level of T1–T3). However, tracheal diverticula may contain fluid, particularly in patients with cystic fibrosis. Depending on the protein content of the fluid, tracheal diverticula may be misinterpreted as a cyst or a lymph node, which carries implications in the oncologic setting ( Fig. 3 ). Although most patients are asymptomatic, some may rarely present with cough owing to pulmonary aspiration or pneumonia, because the diverticula may serve as a reservoir for food residue or secretions. ,

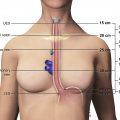
Bronchogenic cysts result from abnormal budding of the primitive foregut, which also gives rise to the tracheobronchial tree, during embryologic development. These benign lesions may arise from any mediastinal compartment, but typically occur in the visceral compartment near the carina or, less commonly, the right paratracheal region. Histologically, bronchogenic cysts are lined by respiratory epithelium and contain a thick mucoid material. Bronchogenic cysts can grow substantially in size without causing symptoms; however, patients may report symptoms when adjacent mediastinal structures are compressed. Bronchogenic cysts typically manifest as well-defined lesions composed of simple fluid. However, when CT attenuation of fluid with a high protein content approaches that of soft tissue, bronchogenic cysts may be misinterpreted as neoplasms. MR imaging adds value by demonstrating the cystic nature with a high signal intensity on T2-weighted images. The signal intensity on T1-weighted images varies depending on the cyst contents ( Fig. 4 ).