KEY POINTS
The embryonic period (the first 8 postfertilizational weeks) is subdivided into 23 morphological stages, which, because they are based on internal as well as external criteria, cannot be identified with confidence by ultrasonography.
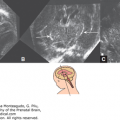
The three major divisions of the brain are found early (stage 9), closure of the neuropores seals the cerebrospinal cavity at 4½ weeks (stage 13), and the five main subdivisions of the brain are visible at 5 weeks (stage 15).
The telencephalon is identifiable already at 4 weeks (stage 10) and begins to diverticulate at 5 weeks (during stage 14), but holoprosencephaly is more than a mere failure of diverticulation and may begin as early as 3 weeks (stage 8), as can cyclopia and anencephaly.
The appearance of the cortical plate (stage 21) heralds the beginning of lamination of the cerebral cortex, the basal nuclei and internal capsule are progressing, and the brain is developmentally advanced at the end of the embryonic period.
Prominent features during the fetal period are the C-shaped structures, including the corpus callosum, and the appearance of sulci and gyri on the cortical surface at the middle of prenatal life.
Prenatal life can be divided conveniently into (1) the embryonic period proper, that is, the first 8 weeks following fertilization, and (2) the fetal period, which extends to birth. The distinction between the embryonic and fetal periods is well founded and has long been established in human embryology. The embryonic period is that time during which new features appear with great rapidity, whereas the fetal period is characterized more by the elaboration of existing structures. Moreover, the vast majority of congenital anomalies appear during the embryonic period. The difference is highlighted by the fact that the embryonic period has been successfully subdivided into morphological stages, whereas the fetal period has so far defied such a procedure.
The embryonic period has been divided into 23 developmental (Carnegie) stages (Table 1-1), which have been listed in detail by O’Rahilly and Müller,1 in whose monograph the early development of the human embryo has been thoroughly described. Each stage, on average, lasts slightly more than 2 days. The stages are based on both external and internal morphological criteria and depend mainly on features that change rapidly, such as the number of somitic pairs, the early appearance of the eye, and the form of the developing limbs. Although it may sometimes be possible to estimate approximately a given stage on ultrasonography, the staging system is based on having an embryo “in the hand” rather than in utero. Moreover, very early as well as late stages can be identified precisely only by histologic examination.
| Embryonic Period | |||
|---|---|---|---|
| Stage | Greatest Length (mm) | Approximate Age (days) | Key Features |
| 1–7 | 0.1–0.4 | 1–19 | Very early embryo |
| 8 | 1 | 23 | Neural folds and groove |
| 9 | 2 | 25 | Mesencephalic flexure; rhombencephalon, mesencephalon, prosencephalon; 1–3 S |
| 10 | 3 | 28 | Fusion of neural folds begins; telencephalon and diencephalon distinguishable; optic primordia; 4–12 S |
| 11 | 3.5 | 29 | Rostral neuropore closes; 13–20 S |
| 12 | 4 | 30 | Caudal neuropore closes; secondary neurulation begins; 21–29 S |
| 13 | 5 | 32 | Closed neural tube; primordium of cerebellum; isthmus rhombencephali; 30–?; Figure 1–2A |
| 14 | 6 | 33 | Pontine flexure; future cerebral hemispheres; all 16 neuromeres present |
| 15 | 8 | 36 | Five subdivisions: medulla, pons, midbrain, diencephalons, telencephalon; Figures 1–2B; 1–3A, B; 1–4 |
| 16 | 10 | 38 | Thalamus; Figure 1–5 |
| 17 | 13 | 41 | Internal and external cerebellar swellings; Figure 1–2C |
| 18 | 15 | 44 | Future corpus striatum; interventricular foramina defined; Figure 1–6 |
| 19 | 17 | 46 | Choroid plexus of fourth ventricle |
| 20 | 20 | 49 | Choroid plexus of lateral ventricles; Figure 1–3C, D |
| 21 | 23 | 51 | Anterior and inferior horns of lateral ventricle; circulus arteriosus complete; Figure 1–2D |
| 22 | 26 | 53 | Internal capsule |
| 23 | 29 | 56 | Caudate nucleus and putamen; anterior commissure begins |
| Fetal Period | |||
| Trimester 1 | Cerebellar halves unite, and vermis becomes defined Corpus callosum is still very limited Aqueduct appears narrow Posterior horn of lateral ventricle | ||
| Trimester 2 | Corpus callosum covers roof of third ventricle Sulci and gyri become visible on hemispheric surface Crura cerebri are prominent Hippocampal formation becomes S-shaped Myelinization begins in CNS | ||
| Trimester 3 | Insula buried by opercula | ||
Although schemes have been devised to subdivide the fetal period according to either measurements or age (one of the simplest, into trimesters, is still very useful), no morphological staging system is available, largely because changes are neither sufficiently rapid nor adequately spectacular.
It should be pointed out that, in current usage in anatomy, practically all eponyms (eg, Luschka, Magendie, Monro, Reil, Rolando, and Sylvius) are now obsolete because they convey nothing concerning either the site or the nature of the relevant structure. The addition “of Monro” or “of Sylvius” is superfluous because there is only one interventricular foramen and only one aqueduct in the brain.
Several different measurements, such as the biparietal diameter and the ossified femoral length, are very important in later development, but the most useful datum throughout prenatal life is still the greatest length, exclusive of the (flexed) lower limbs.2 Crown-rump (C-R) length is less satisfactory because point C, which overlies the midbrain, is frequently difficult to locate, and point R is imprecise. The greatest length, which is independent of fixed points, is much simpler to ascertain and is in fact what is generally measured.3,4
The distinction between stages and measurements should be kept clearly in mind. The “18-mm stage” is incorrect usage, because 18 mm is merely a length, not a stage as the term is used in embryology.
Just as postnatal age commences at birth, prenatal age begins at fertilization. Ovulation is sufficiently close, so that the term postovulatory has frequently been used to indicate age. Particularly since the advent of in vitro fertilization, however, age is best referred to as postfertilizational.
When a reliable menstrual history is available, or indirectly by the conventional addition of 2 weeks to the age, the duration from the first day of the last menstrual period (LMP) may be used. The duration is expressed in (post) menstrual weeks and days. This is perfectly acceptable provided that the duration is designated as (post)menstrual, and it is not referred to as age. At postmenstrual week 1, an embryo does not even exist.
In summary, two systems of designation are available: (1) age, which is postfertilizational and generally estimated; and (2) (post)menstrual duration, which, although it is not age (and should not be combined with that word), is a useful guide in clinical practice. The type of weeks being used should be specified: postfertilizational weeks or weeks of age, or (post)menstrual weeks. Confusion is thereby eliminated.
It has been shown that the term gestational age in the literature is either not defined or is used indiscriminately for postmenstrual weeks and days or for postfertilizational age.5 The continuing confusion concerning prenatal age is unnecessary and disappears once the ambiguous and superfluous term gestational age is abandoned.
The advent of ultrasonography has led to the construction of many elaborate tables of prenatal measurements related to prenatal age, or more generally to intervals since the LMP. Although not all of these tables are in agreement in detail, it would be agreed that at the end of the embryonic period, when the greatest length is ~30 mm, the age is 8 weeks, corresponding to 10 postmenstrual weeks.
The development of the human nervous system is summarized here (Tables 1–1 and 1–2). The most detailed and precise account of the prenatal human brain, with particular emphasis on the embryonic period, is The Embryonic Human Brain: An Atlas of Developmental Stages.6 That study contains a bibliography of 350 entries, and therefore a detailed list of references is not provided here. Some significant features have been summarized recently.7
| Postfertilizational Weeks (Age) | Postmenstrual Weeks | Features |
|---|---|---|
| 5 | 7 | First indication of brain |
| 5–6 | 7–8 | Cerebral hemispheres, lateral ventricles, wide interventricular foramina Future third ventricle Mesencephalon and future aqueduct Rhombencephalon and fourth ventricle |
| 6–7 | 8–9 | Choroid plexuses of lateral ventricle Telencephalon, diencephalon, mesencephalon, metencephalon, myelencephalon Cerebellum, pontine flexure, lateral recesses of fourth ventricle |
| 7–8 | 9–10 | Falx cerebri C-shaped lateral ventricles Narrower third ventricle Isthmus prosencephali (between third ventricle and aqueduct) |
| 8–10 | 10–12 | Growing thalami Cerebellar hemispheres seem to meet Cerebellar peduncles Tentorium cerebelli Fourth ventricle divided by choroid plexus |
The CNS arises mostly from a part of the ectoderm known as the neural plate. The folding of the neural plate to form successively the neural groove and the neural tube is termed primary neurulation and is the first visible sign of the nervous system. It begins when the embryo is ~1 mm in length. Closure of the neural groove begins near the junction of the future brain and spinal cord. The still open ends of the developing neural tube are known as the rostral and caudal neuropores, which close successively at about 4 weeks. The closure of the rostral neuropore is bidirectional: rostrocaudal and caudorostral. Although small and variable accessory loci of fusion of the neural folds may sometimes be seen, a specific pattern of multiple sites of fusion, such as has been described in the mouse, does not occur in the human.8 Moreover, attempts to force the classification of neural tube defects into such a pattern are unconvincing. Primary neurulation is completed by the separation of neural from surface ectoderm by the interposition of mesenchyme.
In diastematomyelia the spinal cord is partially split longitudinally into right and left halves that are separated by a fibrocartilaginous or bony spur in the vertebral canal. This may be a manifestation of the split notochord syndrome, which is generally attributed to persistence of the neurenteric canal, a temporary communication through the primitive node (at stages 8 through 10).
Anencephaly, a partial absence of the brain and the overlying cranial vault, frequently arises from (1) failure of the rostral neuropore to close, followed by (2) protrusion of the brain (exencephaly), and finally (3) degeneration of the exposed portions.9 The defect arises early (probably stages 8 and 9), before postfertilizational week 4, and defective production of mesenchyme is considered to be of fundamental importance.10
Holoprosencephaly is a variable deficiency in “diverticulation” of the prosencephalon to form the cerebral hemispheres and is usually accompanied by facial malformation. Abnormal induction by an area of the future forebrain near the prechordal plate very early (stages 7 and 8) is believed to be significant.11 The failure of lateralization may be complete (alobar holoprosencephaly), partial (semilobar), or only rostral (lobar).
Cyclopia is the occurrence within a single orbit of a median eye (sometimes distinguished as synophthalmia) or paired ocular structures.11,12 The defective lateralization (which is not a fusion) is believed to be caused by the lack of normal inhibition of the median portion of the originally single optic field.6 Normally, the prechordal plate induces paired optic primordia very early (stages 7 and 8).
Encephalo(meningo)celes are generally in the occipital region. They are covered by skin and hence are believed to arise after closure of the neural tube, probably in the presence of mesenchymal insufficiency. Those situated anteriorly (eg, in the fronto-ethmoidal region) constitute a separate category and are based on a primary disturbance in the separation of neural and surface ectoderm at the site of final closure of the rostral neuropore, resulting secondarily in a mesodermal defect at this site.13
Secondary neurulation is the continuing formation of the sacrococcygeal part of the spinal cord from the caudal eminence, without direct involvement of the surface ectoderm (neural plate). It begins once the caudal neuropore has closed. The transition from primary to secondary neurulation is at the site of closure of the caudal neuropore. The caudal neuropore closes at the level of somitic pair 31, which corresponds to future vertebral level S2 in the embryo. Because of the ascent of the spinal cord during the fetal period, however, the site of the former caudal neuropore ascends also and corresponds to a higher vertebral level postnatally.
As the embryo becomes more elongated, the neural groove becomes deeper, and the three major divisions are distinguishable in the folds of the completely open neural groove: the forebrain, the midbrain, and the hindbrain. The site of the midbrain is indicated by the mesencephalic flexure, which remains distinct throughout the embryonic period (Figure 1–1). The neural tube has not yet formed, and the “brain vesicles” are largely a myth. The forebrain soon becomes subdivided (earlier than previously appreciated) into the diencephalon and the telencephalon medium. The embryo is now ~3 mm in length.
Figure 1–1.
Right lateral views of the brain at postmenstrual weeks 6 to 8 (4 to 6 weeks of age). The midbrain is shaded, and the mesencephalic flexure (short arrow) is evident. The beginning of the pontine flexure can be seen (larger arrow). The optic cup and the internal ear are included. (Reproduced from O’Rahilly and Müller, 2006,6 with permission).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree