Introduction
Radionuclide myocardial perfusion imaging (MPI) plays a key role in the diagnosis and management of individuals with known or suspected coronary artery disease (CAD). Recent breakthroughs in single-photon emission computed tomography (SPECT) technology and software, along with the increasing availability of positron emission tomography (PET) scanners, are rapidly changing the existing paradigm of MPI. Cardiac SPECT MPI is a versatile tool to diagnose obstructive CAD noninvasively; it has a sensitivity of 82% to 88% for exercise and 88% to 91% for pharmacologic stress and a specificity of 70% to 88% for exercise and 75% to 90% for pharmacologic stress. Perfusion defect size, severity, location, and reversibility are powerful markers, not only to identify the extent and severity of CAD, but also to estimate the risk of future myocardial infarction and cardiac death. The left ventricular ejection fraction (LVEF) and volumes are critical to guide patient management. Cardiac PET MPI is more accurate than SPECT MPI to diagnose obstructive CAD. Peak stress ejection fraction (EF) by PET provides significant incremental value over perfusion imaging to identify obstructive multivessel CAD. Quantitative PET with myocardial blood flow and coronary flow reserve (CFR) are powerful tools to stratify risk, exclude multivessel CAD, and identify coronary microvascular dysfunction. Hybrid PET-CT imaging with calcium scores and CT coronary angiography can significantly enhance the diagnostic value of quantitative MPI. Multiple factors, however, can compromise the quality of study, affect the eventual scan interpretation, and thereby affect patient management and outcomes. In this chapter, we will address the challenges of stress MPI before and during the test and during reporting, along with solutions to optimize radionuclide MPI.
Before the Test: Appropriate Patient Selection
Rest myocardial blood flow may remain normal, even with severe underlying coronary stenosis, due to compensatory coronary microvascular vasodilation. Therefore detection of inducible ischemia with exercise or pharmacologic stress remains the cornerstone in the diagnosis of CAD. Whereas exercise and dobutamine stress increase myocardial oxygen demand, vasodilator stress agents produce differential hyperemia between the diseased and nondiseased territories. All forms of stress testing have inherent risks, which can be minimized by appropriate selection of patients and stress tests.
Challenge: Patient Selection
Appropriate patient selection is critical to ensure the optimal risk versus benefit of MPI, including the type of stress test. As radionuclide MPI is rapidly expanding with conventional SPECT, novel semiconductor detector SPECT, and PET MPI, referring physicians may find it challenging to identify the right test for a given clinical question. Radionuclide MPI and the more recent, multisocietal, appropriate use criteria (AUC) documents provide valuable information regarding the clinical use of radionuclide imaging—SPECT and PET are used interchangeably in the AUC document. The RAND Corporation has defined appropriate use as one wherein the expected clinical benefit of the test outweighs the risks of the procedure, including the radiation risk. The radionuclide AUC were developed based on expert opinion, with the test indications categorized as appropriate, may be appropriate (previous term uncertain ), and rarely appropriate (previous term inappropriate ) by an expert panel. Several clinical studies have confirmed that although most MPI studies (70%–85%) are performed for appropriate indications, a smaller but significant proportion of tests is performed for may be appropriate or rarely appropriate indications. To reduce the use of stress testing in the latter two categories, the Choosing Wisely initiative of the American Board of Internal Medicine (ABIM), in conjunction with Consumer Reports , has listed specific recommendations for physicians and patients to be aware of prior to performing a study; it is summarized as follows:
- 1.
Don’t perform stress cardiac imaging or coronary angiography in patients without cardiac symptoms unless high-risk markers are present.
- 2.
Don’t perform cardiac imaging for patients who are at low risk.
- 3.
Don’t perform radionuclide imaging as part of routine follow-up in asymptomatic patients.
- 4.
Don’t perform cardiac imaging as a preoperative assessment in patients scheduled to undergo low- or intermediate-risk noncardiac surgery.
- 5.
Use methods to reduce radiation exposure in cardiac imaging, whenever possible, including not performing such tests when limited benefits are likely.
Potential Solutions
A careful review of the reason for the study will ensure appropriate patient selection. This may sometimes involve a discussion with the referring physician to clarify that the clinical question can be appropriately answered by radionuclide MPI or any alternative tests.
An online app (radionuclide appropriateness use criteria) is helpful to evaluate the AUC for any given indication. As of January 2017, Medicare payments have been linked to documentation of AUC in the study report. Several decision support systems now incorporate professional societal AUC criteria into physician order entry systems.
Stress Test: Exercise and Pharmacologic Stress
Exercise is physiologic and preferred in patients able to perform routine daily activities. Exercise stress also provides information on functional capacity and symptoms, and is a powerful prognostic marker. Treadmill exercise with a Bruce or modified Bruce protocol is the most common type of stress test in the United States; bicycle stress tests are more commonly used in Europe. Although functional testing has good diagnostic accuracy, nonobstructive disease may not be detected, especially if the test is submaximal. Exercise stress alone, without imaging, is appropriate in low- or intermediate-risk patients with an interpretable electrocardiogram (ECG). Even in patients who need imaging, those with moderate physical functioning should undergo a symptom-limited exercise stress test. Pharmacologic stress testing is an excellent alternative in patients who are unable to exercise, have contraindications to exercise stress, or achieve a submaximal target heart rate during exercise.
Imaging in these patients is driven by other patient characteristics, such as intermediate to high risk for CAD, known CAD, or a baseline ECG that makes ischemic changes uninterpretable. Incremental diagnostic and prognostic information is obtained from the exercise from parameters such as the Duke treadmill score and heart rate recovery. For a standard Bruce protocol, the Duke treadmill score is calculated as exercise duration in minutes minus five times the maximal ST segment depression (in millimeters), minus four times the chest pain score (0 = no angina, 1 = nonlimiting angina, and 2 = exercise-limiting angina), and heart rate recovery as heart rate at peak exercise minus heart rate at 1 minute into recovery (normal >12 beats/min). A mildly abnormal SPECT MPI portends a higher risk in patients with an intermediate or high Duke treadmill score than in patients with a low Duke treadmill score.
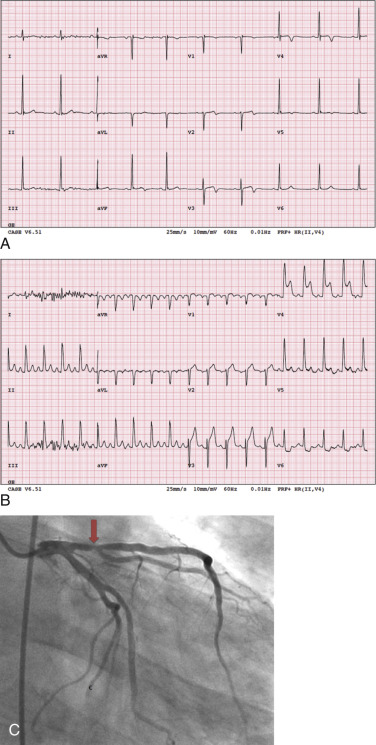
Challenge: Rest Chest Pain
Occasionally, patients referred for a stress test may experience rest chest pain at the time of the test.
Potential Solutions
Examine the patient clinically, ensure hemodynamic stability, and obtain a rest ECG. If clinically unstable, refer the patient to the emergency room for further evaluation.
If the patient has ongoing chest pain but is stable hemodynamically, consider injection of a perfusion tracer (SPECT or PET) during chest pain and rest imaging. In a randomized clinical trial of patients presenting with chest pain to the emergency room, rest MPI, with radionuclide injected during ongoing chest pain or within 3 hours of pain resolution, significantly reduced unnecessary hospitalizations without differences in clinical outcomes. The extent of perfusion abnormality correlated well with the anatomic extent of myocardial ischemia. The sensitivity and specificity of rest MPI performed at <6 hours of the onset of symptoms are >90% and 80%, respectively. However, the reduced specificity is due to lack of differentiation between acute reduction in blood flow versus hibernation or a chronic infarct. The negative predictive value of a completely negative rest MPI in patients with acute chest pain is >99%. Furthermore, resting MPI in these patients has prognostic clinical implications. A positive resting MPI has up to a 30% short-term event rate as opposed to a <1% 30-day event rate for a normal MPI.
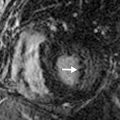
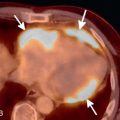
If the patient is clinically stable and the rest MPI (during chest pain) is normal, the next step is to proceed with the stress test and imaging. If the rest MPI is abnormal and the patient is clinically unstable, referral to coronary angiography may be warranted. If the patient is clinically stable and has no active chest pain, 18 F-fluorodeoxyglucose (FDG) PET can be performed. A mismatch between rest myocardial perfusion and FDG metabolic imaging suggests hibernating myocardium as a potential cause for the rest angina ( Fig. 11.1 ).
Exercise Stress Testing
A Bruce or modified Bruce protocol with 3-minute stages is the most commonly used exercise treadmill protocol. During the treadmill test, the ECG is monitored, blood pressure is obtained at regular intervals, and symptoms are assessed. The physician performing the test should be aware of the indications for early termination of the exercise stress. Unlike stress echocardiography (echo), where the exercise is terminated at maximal exercise, the exercise during MPI should be continued for at least 1 minute after injection of the radiotracer to ensure adequate radiotracer circulation and myocardial uptake.
Challenges During Exercise Stress Testing
During the stress test, patients can experience chest pain, hypotension, ischemic ST segment changes, tachyarrhythmias or bradyarrhythmias, syncope, falls, or other complications.
Potential Solutions
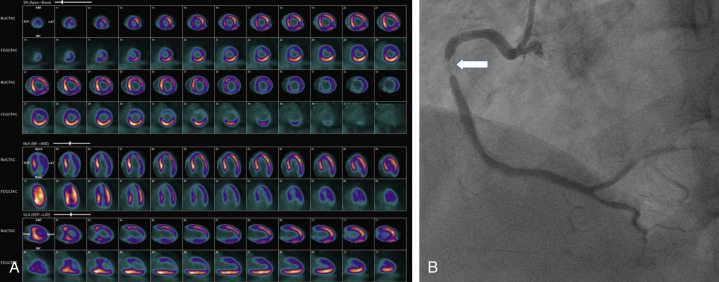
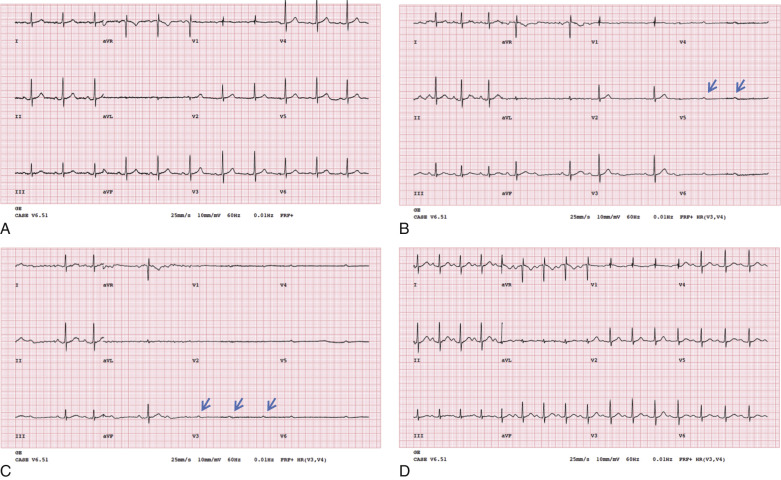
Objective evidence of significant ischemia on the ECG, horizontal or downsloping ST depression (>2 mm), and significant ST elevation (>1 mm; Fig. 11.2 ) are reasons to inject the radiotracer (if feasible) and terminate the exercise, even if the patient is asymptomatic and the age-predicted maximal heart rate (85% of 220 minus age in years) has not been achieved. When significant ST segment elevation is noted during exercise, it localizes the ischemia to a coronary territory. Therefore radiotracer injection and imaging are typically avoided and the patient scheduled for emergent coronary angiography—unless the patient is stable and there is a delay for coronary angiography.
Subjective complaints of moderate to severe chest pain during exercise may be reasons to terminate exercise. In a patient experiencing exercise-limiting chest pain, if the heart rate is submaximal, the exercise test is terminated, without radiotracer injection. A vasodilator study is performed after the chest pain resolves. Reproducible angina during stress, even in the absence of ECG changes, may warrant additional workup. In hemodynamically stable patients, sublingual nitroglycerin can be administered for moderate to severe chest pain. Persistent or worsening chest pain, even after three doses of sublingual nitroglycerin (0.4 mg) at 5-minute intervals, or hemodynamic instability may be a reason for referral to urgent coronary angiography.
Hypotension, a decrease in systolic blood pressure below the preexercise standing blood pressure, especially when accompanied by other ischemic signs or symptoms, is a marker of underlying significant CAD and death. Exercise-induced hypotension is typically managed by IV fluids and, if necessary, referral to coronary angiography. Nitroglycerin and beta blockers are contraindicated in patients with exercise-induced hypotension.
Symptomatic arrhythmias are managed as per the American Heart Association’s advanced cardiac life support algorithms. Falls or syncope should be addressed immediately with evaluation of hemodynamic status, arrhythmias, and orthopedic limitations and assessment of injuries.
Submaximal Heart Rate Response
Chronotropic incompetence is defined as the inability to attain 85% of the age-predicted maximal heart rate during exercise. It may be related to cardiac disease, medication use (e.g., beta blockers, calcium channel blockers), or orthopedic limitations. The diagnostic accuracy of MPI to identify significant obstructive CAD is limited in patients with a submaximal heart rate response. Therefore if the reason for the MPI is to assess response to medical therapy (as in medically managed patients with CAD), radiotracer may be injected at the heart rate achieved despite submaximal heart rate, especially if a workload of 5 METs (metabolic equivalent of task [or just metabolic equivalent]) is achieved. However, if the reason for the study is to identify ischemia or understand the hemodynamic significance of stenoses of indeterminate significance or preoperative evaluation, a maximal heart rate response is preferred for administration of the radiotracer. Whatever the reason, chronotropic incompetence is a marker of high 2-year mortality, particularly when associated with perfusion defects on MPI. The reasons for high mortality with chronotropic incompetence are not known but may reflect cardiac autonomic function, which may be evident in individuals with advanced heart disease.
Potential Solutions
In patients with submaximal heart rate response to exercise, pharmacologic stress testing (with a vasodilator or dobutamine) is an excellent alternative. Adenosine and dobutamine infusions are typically administered after the treadmill test is terminated. Regadenoson, a novel A2A receptor agonist, is supplied as a prefilled syringe and is not weight-based but can be administered during standard Bruce treadmill protocol when the heart rate is noted to be submaximal, followed by radiotracer injection. When used appropriately, regadenoson with treadmill exercise is safe and yields a result similar to that with vasodilator stress without exercise.
Pharmacologic Stress Testing: Vasodilators and Dobutamine
Adenosine, dipyridamole, and regadenoson are the most commonly used vasodilators for stress MPI. Adenosine and dipyridamole are nonselective adenosine receptor agonists (bind to adenosine A1, A2A, A2B, and A3 receptors), whereas regadenoson is a selective adenosine A2A receptor agonist. In addition to general contraindications for stress testing, vasodilators are contraindicated in patients with sinus node dysfunction or high-degree atrioventricular (AV) block without a functioning pacemaker, active wheezing, oxygen-dependent chronic obstructive lung disease, recent use of methylxanthines (within 12 hours) or oral dipyridamole (48 hours), and systolic blood pressure <90 mm Hg. Administration of IV adenosine to individuals who are on oral dipyridamole (sometimes used in patients with transient ischemic attacks) can result in prolonged heart block and hemodynamic collapse. Dobutamine infusion is an alternative for patients with contraindications to vasodilator stress and patients with caffeine or oral dipyridamole intake. Dobutamine is preferred mainly in patients with active reactive airway disease and is avoided in patients with acute myocardial infarction, uncontrolled atrial or ventricular tachyarrhythmias, uncontrolled hypertension, aortic dissection, or a large aortic aneurysm (>6 cm).
Challenges During Pharmacologic Stress Testing
In carefully screened and selected individuals, adverse effects during pharmacologic stress testing are uncommon. Severe chest pain, ischemic ECG changes (ST elevation >1 mm in non–Q wave leads or >2-mm ST depression in three consecutive beats), hemodynamic instability (decrease in systolic blood pressure <90 mm Hg), signs of poor perfusion (e.g., pallor, cool extremities), high-degree AV block ( Fig. 11.3 ), wheezing, atrial or ventricular tachyarrhythmias or bradyarrhythmias, or intolerance to the infusion have been reported. Low-grade exercise with vasodilator infusion can attenuate side effects and improve image quality by reducing the subdiaphragmatic uptake of the tracer.
Potential Solutions
Mild side effects are common and usually well tolerated. Moderate to severe adverse effects during adenosine or dobutamine infusions may necessitate premature termination of the infusion.
Infusion of regadenoson (administered as a bolus over 10 seconds) or dipyridamole (administered over 4 minutes but with symptoms occurring at 7 minutes) typically is not terminated, but the hyperemia is counteracted by IV aminophylline.
Aminophylline (50–150 mg slow IV push, 1–1.5 mg/kg) given as a slow IV bolus over 60 to 90 seconds can be used to reverse the hyperemic effects of vasodilator agents. Due to the short half-life of adenosine, aminophylline is not typically needed unless the patient has wheezing or prolonged heart block, despite termination of the adenosine infusion.
For hypotension, IV fluids may be administered (100–150 mL of normal saline IV as a rapid bolus), followed by IV aminophylline, as described above.
Chest pain typically resolves with termination of the infusion agent and after IV aminophylline. If chest pain persists, especially with ST depression, sublingual nitroglycerin, 0.4 mg, can be administered, provided that the systolic blood pressure is >90 mm Hg and there is no recent history of the use of sildenafil or other phosphodiesterase inhibitors. IV beta blockers (5 mg IV metoprolol [Lopressor] over 30 seconds) can be administered for chest pain, especially during dobutamine infusion.
Wheezing is managed with inhaled albuterol via a nebulizer every 10 minutes, as needed. If wheezing does not improve after two units of nebulizer therapy with albuterol have been administered, consider referral to the emergency room.
High-grade AV block, usually transient, is common with IV adenosine infusion, especially following radiotracer injection at 2 minutes into the infusion. Exercise, hand grip, or supine leg exercise typically improves the heart block. In patients with persistent or symptomatic high-grade AV block, adenosine infusion may be prematurely terminated. Persistent or symptomatic heart block, although less frequent after termination of the adenosine infusion or with dipyridamole or regadenoson, is managed with IV aminophylline.
In cases of hemodynamic instability, signs of hypoperfusion, or sustained ventricular arrhythmias, advanced cardiac life support measures are instituted.
Imaging
Attenuation Artifacts
Artifacts can arise during each step of the scan from patient-related factors or from errors during image acquisition, reconstruction, or processing.
The Challenge
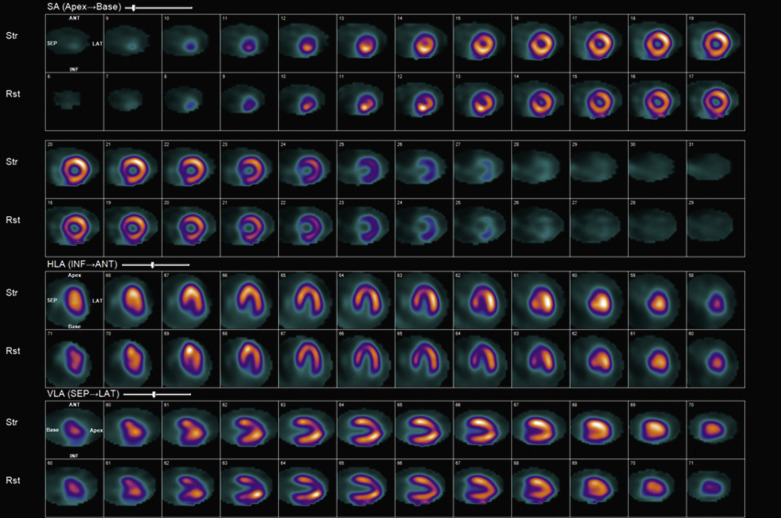
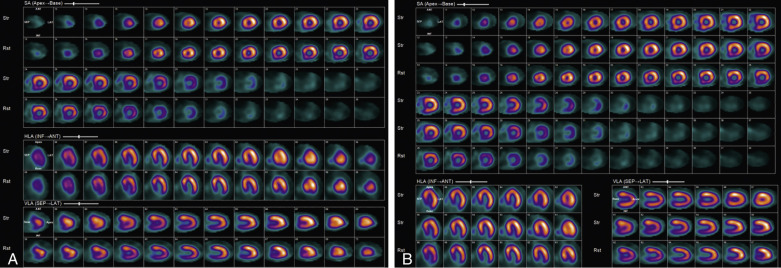
Attenuation of the photons from the patient’s body, typically because of a large body habitus, breast tissue in females, and muscular diaphragm in males, or from arms-down imaging, is a major source of artifact with radionuclide MPI ( Figs. 11.4–11.6 ). Breast tissue typically reduces counts in the anterior or lateral wall of the left ventricle, depending on the size and position of the breasts. Although more often these so-called defects appear fixed, a shift in breast tissue position (especially the left) between the rest and stress scans can produce an apparently reversible perfusion defect (see Fig. 11.4 ). Inferior wall attenuation is seen more commonly in men due to attenuation from a muscular diaphragm (see Fig. 11.5 ).
Identifying the Artifact
A careful review of the raw rotating planar and projection images will help identify attenuation from breast tissue or from the diaphragm. It is also important to identify breast tissue on the stress and rest images to ensure that they are in a similar position.
Potential Solutions
If breast tissue attenuation is the suspected cause of a fixed perfusion defect, gated SPECT, repeat imaging, and attenuation correction can be considered.
Gated SPECT
The advent of technetium-99m ( 99m Tc) perfusion tracers and gated SPECT was a major breakthrough for the differentiation of breast tissue attenuation artifacts from a myocardial scar. Normal regional wall thickening and wall motion confirm the fixed defect as an attenuation artifact, whereas abnormal wall motion confirms a real perfusion defect (hibernation or scar).
Repeat Imaging
If the breast position is different on the rest of the stress images, image acquisition may be repeated with the hope of mitigating the problem. Breast binders are not recommended because they may increase the chance of variable breast tissue attenuation. Imaging in a prone position is helpful for the correction of artifacts related to the diaphragm but can worsen anterior wall attenuation and thus is not recommended.
Higher Radiotracer Dose Imaging
A generalized reduction in the counts resulting from a large body habitus can be mitigated by using a higher radiotracer dose to increase image counts or using 2-day imaging protocols.
With some of the dedicated cardiac SPECT scanners, imaging is performed with the patient in the upright and semirecumbent or supine position. With upright imaging, a breast attenuation artifact results in an inferoapical defect rather than the usual anterior wall perfusion defect. Acquiring stress images with the patient in a supine position, in addition to upright imaging, may help differentiate an artifact from a real defect (seen in both and supine images).
Software
Most commercially available semiquantitative programs compare the perfusion data with gender-specific, normal limits databases and delineate attenuation artifacts. The most specific solution for attenuation artifacts, however, is to measure attenuation and correct for it.
Attenuation Correction
Attenuation correction (AC) is the most direct solution for attenuation artifacts. Soft tissue attenuation can be accurately measured and corrected using a transmission map derived from a radioactive line source, commonly gadolinium-135 ( 135 Gd), with conventional scanners, or using a CT-derived transmission map with hybrid scanners (SPECT CT or PET CT). Currently, the transmission scans are typically sequential to the emission scans (either before or after). Therefore appropriate coregistration between the emission and transmission scans is important. If misregistration is observed, special software is used to realign the images, develop a new attenuation map, and derive a new reconstruction of the emission images, using the revised attenuation map. In cases in which significant attenuation is anticipated, as in patients with a large body habitus or left arm–down imaging, PET MPI may be a preferred alternate study. PET imaging is based on coincidence detection of photons and provides improved spatial and temporal resolution when compared to SPECT. Due to the coincidental detection of photons with PET, attenuated photons are not counted as true counts and may be discarded by the scanners. Hence, attenuation is paradoxically higher, and AC is always used. Compared to SPECT, AC with PET is depth-independent and more robust.
Patient Motion
The Challenge
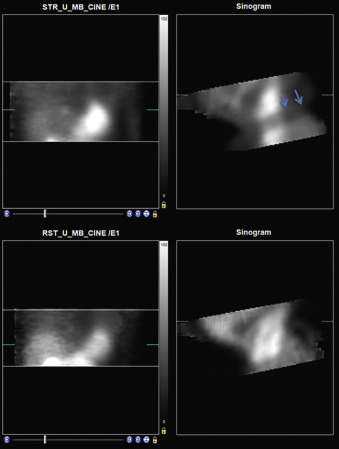
Patient motion during MPI is an important and common source for artifacts with both SPECT and PET MPI. Motion can be vertical, horizontal (side to side), or complex. Motion may also be gradual or abrupt. A 0.5-pixel movement does not cause a detectable defect, whereas a 1- pixel movement could cause a defect that is detectable but may not be clinically significant; motion of 2 pixels or more causes a clinically significant artifact. With the newer cardiocentric scanners, the acquisition is quick and, due to the reduced scan time and more comfortable position, the frequency of patient motion may be reduced. Patient motion during the transmission scan may also be a significant source of artifacts.
Identifying Patient Motion
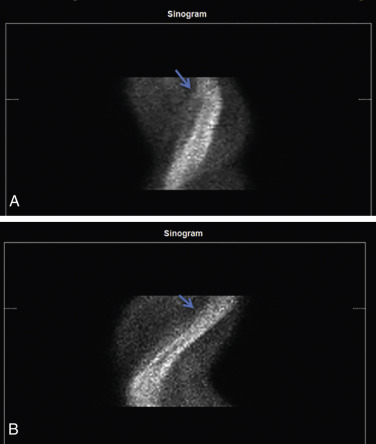
Traditional Anger SPECT Scanners
With traditional SPECT, images are acquired in a temporal sequence using a multiframe acquisition (step and shoot or continuous), with a series of 32 to 34 image frames acquired by each scanner detector, for a total of 60 to 64 frames. A review of rotating projection images can identify patient motion, especially vertical or complex motion. The sinogram may be better at identifying horizontal motion, and a linogram can further provide information about motion ( Fig. 11.7 ).