PROLIFERATIVE HEMANGIOMAS, VASCULAR MALFORMATIONS, AND VASCULAR COMPRESSION SYNDROMES
KEY POINTS
- A clinically useful understanding of hemangiomas and vascular malformations begins with understanding the differences between the two entities and a system for classification of all vascular lesions discussed.
- Proliferative hemangiomas are benign neoplasms that grow faster than the child.
- Malformations grow at the same rate as the patient but may accelerate under the influence of hormones during puberty and pregnancy.
- The concept of the venolymphatic malformation might be used to address that group of lesions in general.
- The complications of these benign entities can be devastating and can mimic the presentation of more ominous pathologic conditions.
- Diagnosis of vascular malformations is almost never difficult.
- Imaging is done to help in the decision of how to best manage often considerable cosmetic and functional problems.
- Vascular malformations are developmental lesions, and they may be syndromic or at least associated with other congenital problems that now are better understood as craniofacial arteriovenous metameric syndrome and cerebrofacial venous metameric syndrome.
GENERAL CLINICAL PRESENTATIONS AND IMPLICATIONS
Proliferative hemangiomas and vascular malformations generally present as discolorations of the skin, indicating their nature as a primarily vascular problem or as a compressible, sometimes waxing and waning, face or neck mass.1–3 Physical findings may vary when these vascular lesions bleed, thrombose, or become inflamed or infected. Deeper lesions may present with functional complaints related to the eye and orbit, mastication, deglutition, speech and hearing, airway problems, and glottic dysfunction. The masses generally present in children and young adults but are sometimes not noticed until middle age. These may also be discovered during prenatal ultrasound or incidentally on imaging studies done for unrelated problems.
Proliferative hemangiomas and malformations most frequently involve the skin. However, mucosal involvement is common, most frequently in the nasal or oral mucosa. Deeper lesions may involve the tonsil, palate, tongue, and neck. Intramuscular malformations (also referred to as intramuscular “hemangiomas”) may also present in the head and neck. Parotid proliferative hemangiomas and malformations are a common cause of parotid mass in pediatric patients and young adults. In infants, deeper lesions may cause airway and feeding problems as well as the usual cosmetic concerns. The laryngeal subglottic vascular malformation is a well-known clinical entity that usually does not require sophisticated diagnostic imaging. Other sites of these lesions include the orbit, mandible, and skull. In the skull, they can mimic the presentation of sinus pericranii.
The main clinical problems with this group of lesions center on their effect on cosmesis and possible functional impairments. These can occur virtually anywhere in the head and neck region as suggested previously, so the related problems come in all sorts of permutations and combinations. Some are associated with syndromes that may pose greater health risk than the vascular origin lesion itself and that must be recognized and reported when these circumstances arise.
The cosmetic effects can be devastating and difficult to manage (Fig. 9.1). The functional consequences can be life threatening if they affect the airway and feeding (Fig. 9.2). Lesser functional problems, although still very important, include those related to the eyes, hearing, and speech. Tooth loss and bleeding related to dental procedures, and other bleeding, occasionally due to an associated coagulopathy, thrombosis, (Fig. 9.3) and ulceration with bleeding or infection (Fig. 9.4), can complicate these lesions. Management is almost never simple when required by one or more of these circumstances. The lesions are usually controlled or marginalized as opposed to completely extirpated by therapy.
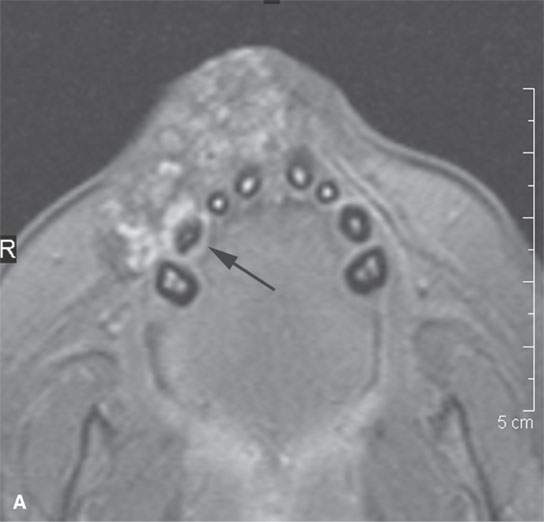
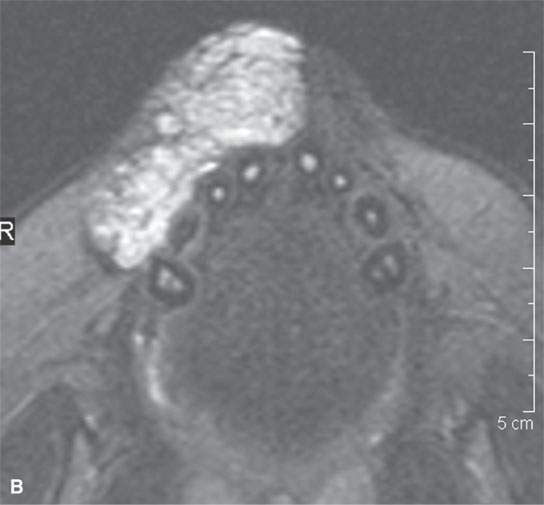
FIGURE 9.1. Proliferative hemangioma of the lip in an infant, producing a considerable cosmetic concern to the parents. A: T1-weighted fat-suppressed image notes that right canine is involved (arrow). B: T2-weighted image of the same patient.
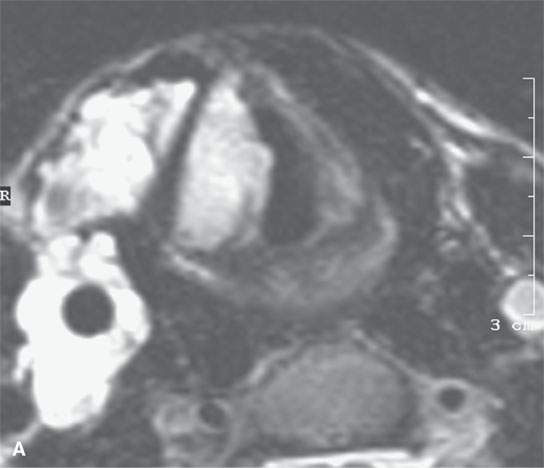
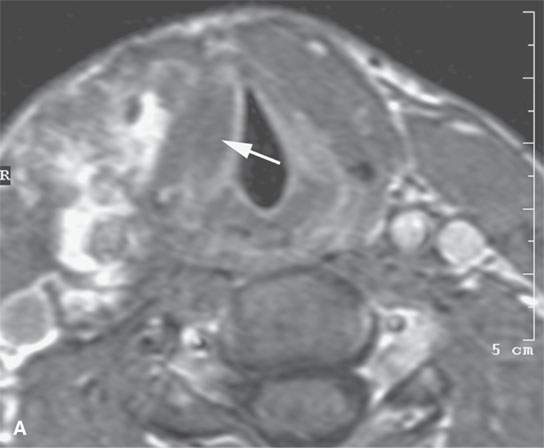
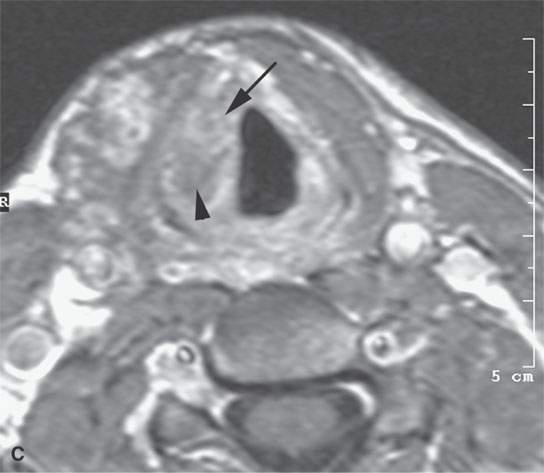
FIGURE 9.2. Magnetic resonance imaging of a venolymphatic malformation of the neck and larynx in a patient presenting with dysphagia and stridor. A: T2-weighted image showing the endolaryngeal component at the false cord level. B: T1-weighted image false cord level shows mixed enhancement pattern with a more (arrow) and a less (arrowhead) enhancing component. C: Contrast-enhanced T1-weighted image at true cord level showing less enhancement (arrow), although delayed images might have shown this component to also be related to slow-flow blood vessel spaces.
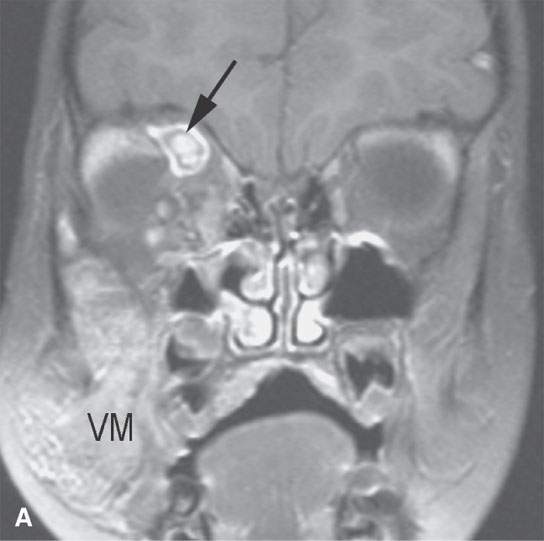
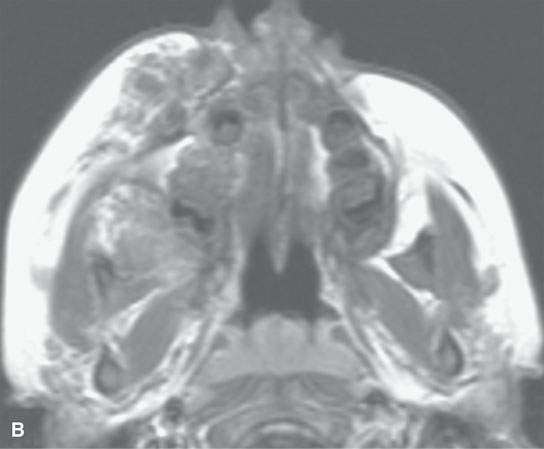
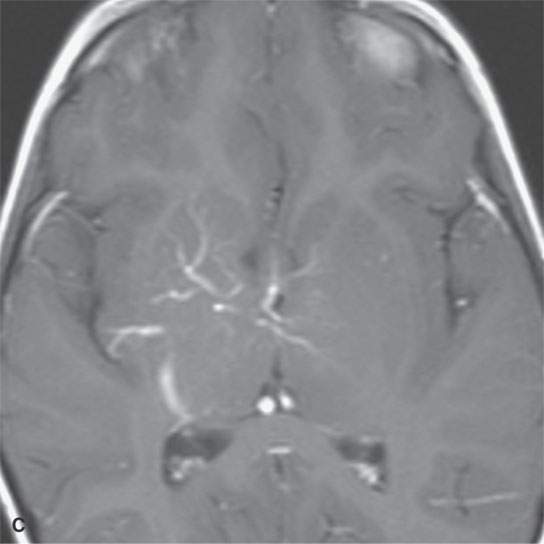
FIGURE 9.3. Magnetic resonance imaging of a venolymphatic malformation that became thrombosed and caused considerable pain and visual problems. A: T1-weighted fat-suppressed contrast-enhanced image showing the thrombosis (arrow). B: T1-weighted contrast-enhanced image. C: Associated anomalous venous drainage pattern in the brain commonly seen in this condition.
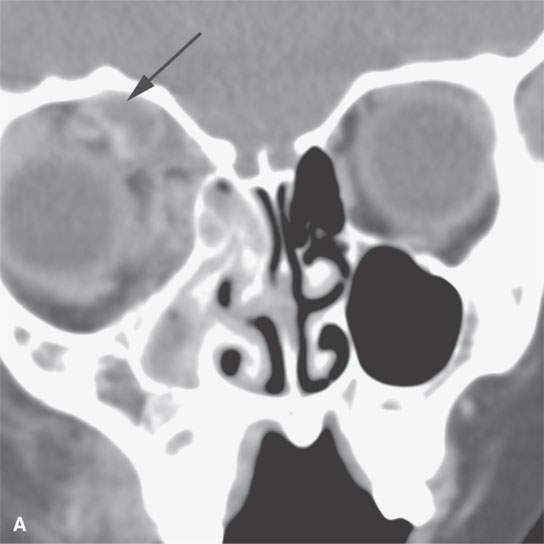
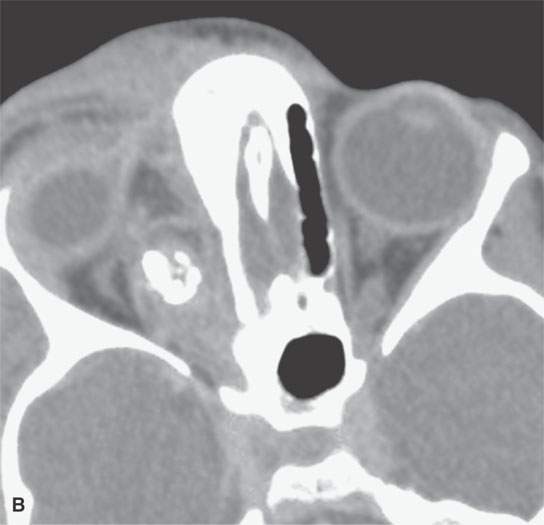
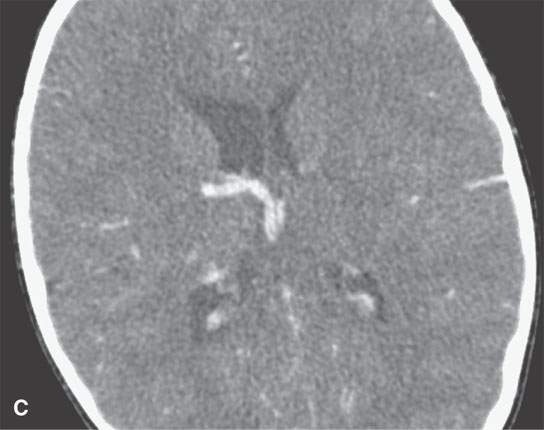
FIGURE 9.4. Contrast-enhanced computed tomography study of a patient presenting with an acutely infected venolymphatic malformation of the orbit. A: Infected component (arrow). B: Axial sections show extensive related cellulitis and phlebolith in the malformation. C: Associated anomalous draining vein.
The malformations and proliferative hemangiomas are typically sporadic but can be associated with other malformations or even be syndromic (e.g., PHACE, Noonan, and cerebrofacial metameric syndromes) (Fig. 9.5).2 Multiple lesions may trigger a search for associated syndromic conditions or similar lesions elsewhere in the body.
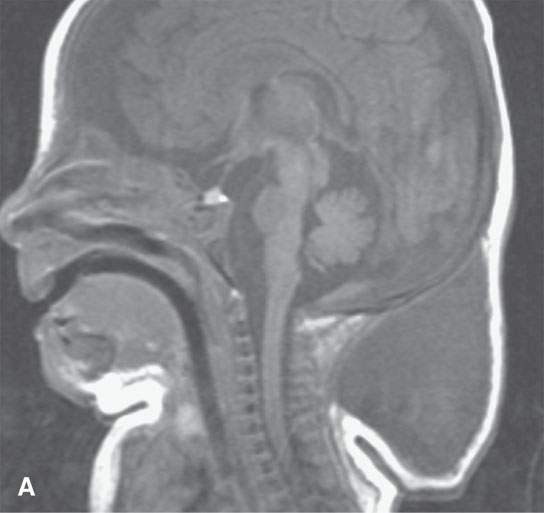
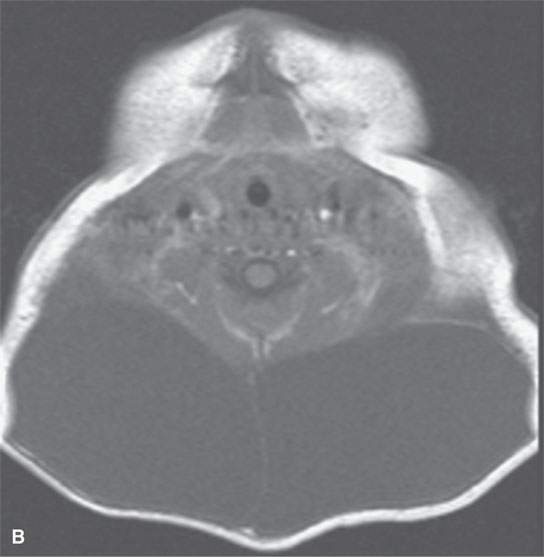
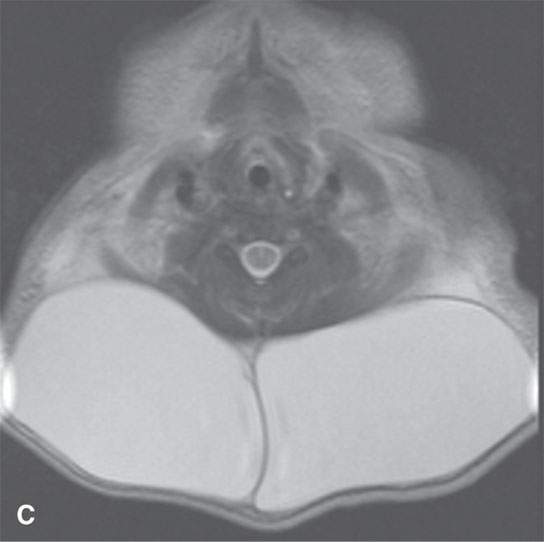
FIGURE 9.5. Non–contrast-enhanced magnetic resonance imaging of a patient with Noonan syndrome and a cystic hygroma–type venolymphatic malformation of the upper neck. A: Sagittal T1-weighted image. B: Axial T1-weighted image. C: Axial T2-weighted image.
CLASSIFICATION
Classification of masses of primary vascular origin presents some difficulty and a confusing array of names for essentially the same problem. Some of these vascular origin masses such as hemangioepithelioma, hemangiopericytoma, and angiosarcoma are true neoplasms and are frankly malignant or have malignant potential; these are considered vascular neoplasms and are discussed separately as such in Chapter 25. Other vascular origin lesions are congenital malformations such as nontraumatic or otherwise acquired atrioventricular (AV) malformations and the venolymphatic malformations such as lymphangiomas and cystic hygromas. There are also acquired traumatic and spontaneous AV malformations and fistulas. Exuberant granulation tissue of the inflammatory response may be mistaken for a vascular malformation or neoplasm if viewed out of its clinical context. Finally, some nonvascular-origin neoplasms of epithelial, mesenchymal, and other origins may have a very prominent vascular stroma or incite a vascular inflammatory response and be mistaken for a vascular malformation or even vascular origin malignancy.
Classification of the congenital/developmental group of these vascular abnormalities has led to some diverse approaches and resultant confusing naming, which are recognized in the following discussion. This work will mainly incorporate the very practical and logical clinical classification devised by Mulliken et al. while recognizing that many still do not adhere to that system—for instance, some preferring to think of some of these lesions as hamartomas. Mulliken et al.’s4 system considers two groups of congenital or developmental origin vascular lesions. The proliferative hemangioma seen in the very young is held as the only true neoplasm in this group and therefore the only “hemangioma.” It is also referred to as a congenital or infantile hemangioma. All other developmental vascular origin lesions are considered malformations.
Pathologists and others may still simply lump developmental vascular masses in one group, such a hamartomas, generically referred to as “hemangiomas” and then divide the hemangiomas into capillary, cavernous, venous, mixed, and hypertrophic categories. This approach primarily is based on the lesion architecture as viewed under the light of a microscope. Overlap of these pathologic vessel types is common. Transition zones are present in many of these lesions, suggesting that each type may represent a stage of progression from a hypertrophic growth phase through capillary to cavernous stages. Another proposed mechanism of evolution is that pulsatile flow transmitted from end arteries may lead to cavernous enlargement of previously capillary-size vessels. The growth pattern seen on imaging studies is in part linked to these general categories regardless of what they are named.
PROLIFERATIVE HEMANGIOMA: INFANTILE AND CONGENITAL
Clinical Perspective and Pathology
It is very useful to consider, as does Milliken, the proliferative hemangioma—a very common lesion in infancy and early childhood—as an entity separate from malformations. The proliferative hemangioma concept is supported by the biologic behavior of the hemangioma, with it being the only one of these lesions that will demonstrate independent, truly neoplastic, growth in cell culture. The natural history of these proliferative hemangiomas is different from the rest of the developmental vascular origin masses in this group, as they grow rapidly in the postnatal period but then most often progressively involute.
Considered generally, the proliferative hemangiomas may be of the skin (dermis) or deeper structures (Figs. 9.1, 1.9, and 6.9). They may then be classified as cutaneous/superficial, subcutaneous/deep, compound/mixed, and visceral.1,2 These lesions tend to grow most rapidly in the first 3 to 8 months of life and then will likely involute by 5 years. If not gone by 7 years, the residua are likely to be permanent. The rapid growth phase may be disturbing to parents and can lead to acute or subacute functional impairment that can become permanent in some cases (Fig. 9.6).
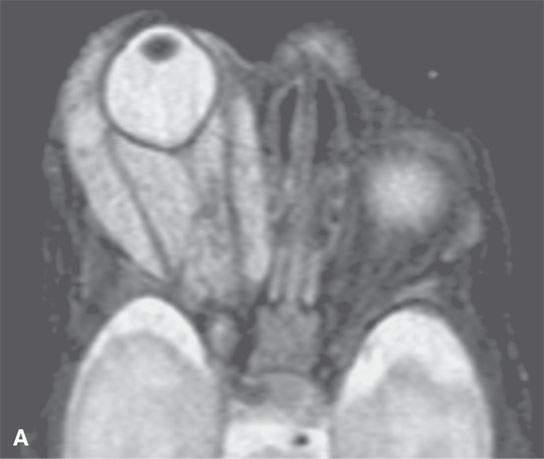
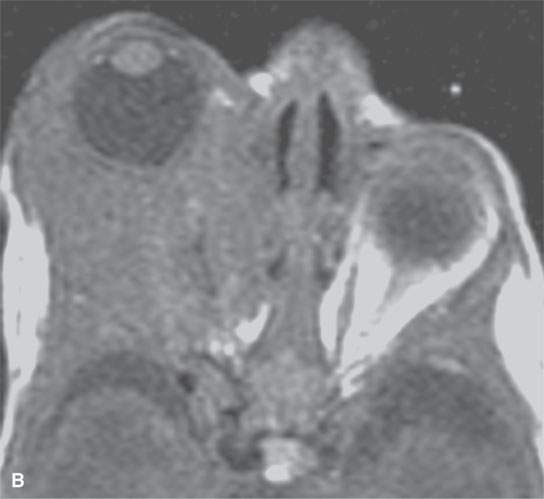
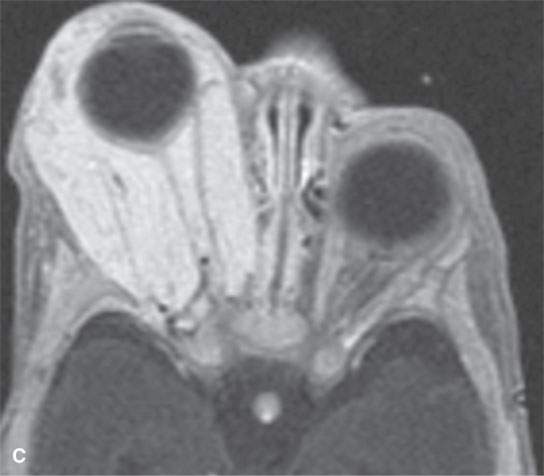
FIGURE 9.6. MR study of an infant who eventually loss the eye to this rapidly proliferative hemangioma that created tension orbit could not be controlled. A: T2-weighted image B: T1-weighted image without contrast C: T1-weighted contrast-enhanced image.
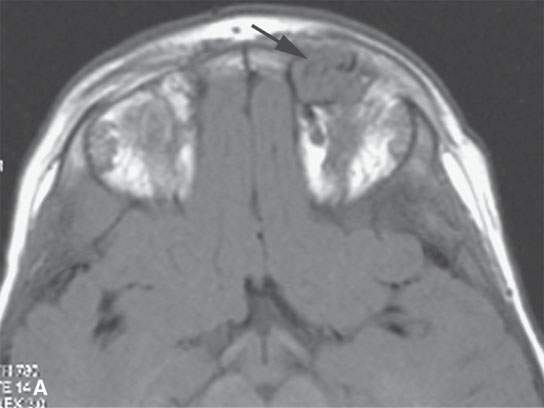
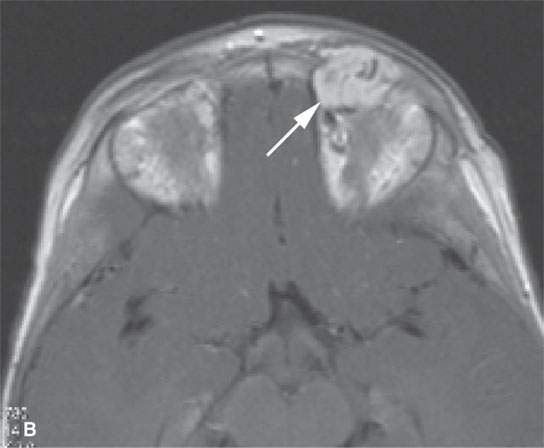
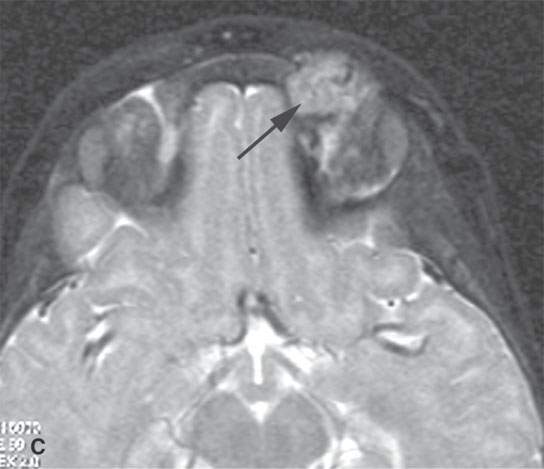
FIGURE 9.7. Magnetic resonance imaging of an orbital proliferative hemangioma. A: T1-weighted non–contrast-enhanced image shows a homogeneous mass with large, tubular associated vessels (arrow). B: T1-weighted contrast-enhanced image shows diffuse, homogeneous enhancement with prominent vessels (arrow). C: T2-weighted image showing a homogenously bright mass consistent with its content of slow-flow vascular spaces.
Considered more specifically, the infantile proliferative hemangiomas may be of the skin or deeper structures. They appear soon after birth and grow rapidly in what is referred to as phase I or the proliferating phase; this is followed by an involuting phase II, usually from age 3 to 5 years, and then an involuted phase III. Cutaneous congenital hemangiomas are present at birth and show a rapid phase of proliferation and enlargement usually followed by involution. They may be referred to as type I, which are rapidly involuting congenital hemangiomas (RICH). Type II may be referred to as noninvoluting congenital hemangiomas (NICH). Some, however, can have a very poor outcome due to uncontrolled rapid growth before they involute (Fig. 9.6). The other developmental masses subsequently referred to as malformations will continue to grow usually in proportion to the individual.
Imaging Appearance
Wherever they occur, proliferative hemangioma is basically a mass composed of many vascular spaces and little stroma. The blood in these spaces is very slow flowing, so it is best considered essentially a microcystic or macrocystic mass. In this way, its appearance on non–contrast-enhanced studies is much like that of benign salivary epithelial lesions or even a microcystic glioma. On TI- and T2-weighted images, hemangiomas are isointense or hyperintense to cerebrospinal fluid (CSF) and well demarcated from surrounding structures (Figs. 9.1, 1.9, and 6.9).2,3,5–7 Internal septations may be visible (Figs. 9.1 and 9.6). Actually, a proliferative hemangioma may be indistinguishable from a venolymphatic malformation on computed tomography (CT) or magnetic resonance (MR) unless contrast is given. Contrast-enhanced computed tomography (CECT) and contrast-enhanced magnetic resonance (CEMR) will usually show marked, diffuse, and relatively homogenous enhancement while the contrast pools in the microcystic vascular spaces during the proliferative phase (Figs. 9.6 and 9.8). Enlarged draining veins both within and outside the hemangioma, depending on the phase of evolution, together with this internal morphology will usually differentiate this from the vascular malformations on the basis of imaging alone. This appearance is seen in the proliferating phase. Phleboliths are not often, if at all, present in these lesions. During regression, large flow voids will diminish and disappear, and nonenhancing areas may be present as a result of thrombosis (Fig. 9.1). Other regressive changes may include calcification, fat, and fibrous replacement.
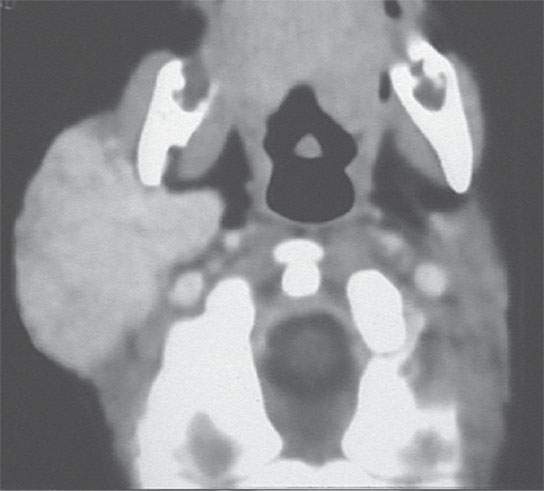
FIGURE 9.8. Contrast-enhanced computed tomography showing a proliferative hemangioma in the right parotid gland.
Proliferative hemangiomas will grow as generally well-delineated masses, but they frequently will insinuate themselves around deeper anatomic structures and within the deep spaces of the face and neck, such as the buccal and masticator spaces. More typically, they will tend to involve the superficial musculoaponeurotic system (SMAS) when they are of the subcutaneous/superficial type (Fig 9.1). The subcutaneous/deep type will grow both deep and superficially along the musculoaponeurotic system and the superficial fascia. The visceral type may remain within the capsule of a gland such as the parotid (Fig. 9.8). Any combination of these patterns is possible, but the biologic behavior, appearance, and natural history will remain the same. The growth pattern influences treatment options.
Treatment
Treatment of proliferative hemangiomas usually involves watchful waiting for the inevitable involution. Serious functional problems or complications such as ulceration or infection may necessarily lead to interventions. Steroids are effective in the proliferative phase.
Immunotherapy, laser, and other surgical excision and obliterative percutaneous interventional radiology approaches may all be employed strategically depending on the clinical goals and extent of the mass (Fig. 9.6).8 Since imaging is critical to determining possible functional compromise and assessing the depth of involvement, bulk, and threat to vital structures, the report must anticipate these circumstances. Since the diagnosis is almost always known in advance, “differential diagnosis” is usually of secondary interest in this disorder.
MALFORMATIONS
General Clinical Perspective and Pathology
The nonproliferative malformations fit relatively well into a pathologic morphologic classification that includes capillary, venous, cavernous, lymphatic, arterial, and combined categories. These differences are usually suggested by imaging studies. These are further usefully classified into low-flow and high-flow categories; this distinction is usually clear on diagnostic imaging exams.
Since there is usually no rapid growth phase, except one that may be triggered by puberty or pregnancy, the problems become ones that are managed with a long-term view. In general, these lesions grow in proportion to the growth of the patient. Rapid enlargement is usually related to bleeding into the lesion, thrombosis with related edema, or infection (Figs. 9.3 and 9.4). The management issues center on preservation of vital and other important functions and very often on cosmesis as described earlier.
The understanding of some craniofacial vascular malformations is now also governed by an improved understanding of their embryologic origin. A system of metameres governs the relationship of neural crest cell migration relative to related parts of the brain and face. Depending on the metamere involved, a predictable segment of the craniofacial continuum will be affected. These disorders of vascular development are now grouped as part of a craniofacial arteriovenous metameric syndrome (CAMS) or cerebrofacial venous metameric syndrome (CVMS). This relatively new knowledge explains the linkage of what sometimes appear to be discontinuous facial and intracranial malformations as well as well-known associations—for example, the Wyburn-Mason syndrome, which is a CAMS, and the Sturge-Weber syndrome, which is a CVMS.
Slow-Flow Malformations
Abnormalities of Lymphatic Development: Venolymphatic or Lymphaticovenous Malformations, Lymphangiomas, and Cystic Hygromas
It may prove useful to lump all of the primarily lymphatic malformations into the category of venolymphatic malformations and drop the older terminology of cystic hygroma or macrocystic lymphangiomas referring to those with large lymphatic spaces (Fig. 9.5) and lymphangioma referring to those with many small lymphatic spaces or microcystic lymphangiomas (Fig. 9.9). However, this is typically not done in practice, and the lymphatic malformations are generally lumped into the term cystic hygroma.
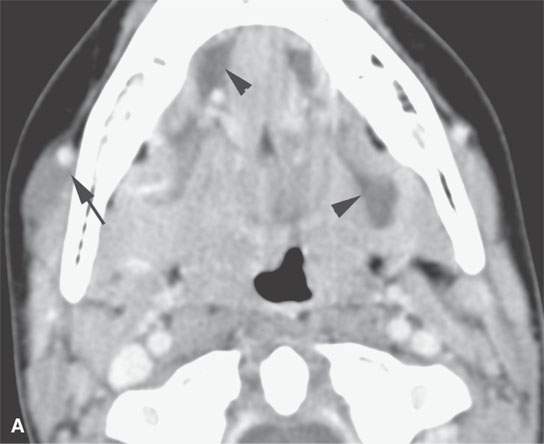
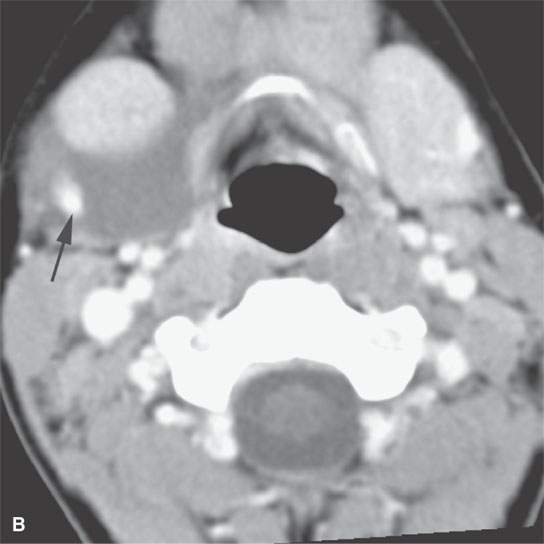
FIGURE 9.9. Contrast-enhanced computed tomography of a lymphangioma of the floor of the mouth and the submandibular space. The lesion was mistakenly believed to be a ranula. A, B: Its true nature is realized by its pattern of insinuation around vessels (arrows) and within existing spaces (arrowheads).
All of these malformations ultimately derive from the venous system, since the lymphatic system is a derivative of the venous system. This lumping suggestion also accounts for the observation that many of what appear to be “lymphatic malformations” will contain capillaries, veins, or cavernous remnants of these maldeveloped blood vessels (Figs. 9.10–9.12). This understanding makes it easier to avoid confusion when some enhancement is seen on imaging studies in what is mistakenly believed to be a purely lymphatic origin malformation or if a malformation enlarges rapidly due to bleeding and fluid–fluid levels and blood products are seen on imaging studies (Fig. 9.13). Since venolymphatic malformations contain lymph elements, they can also enlarge when a local immune response to viral or other infectious agents is mounted. The malformation itself might also become infected (Fig. 9.4). Referring to all of these malformations as venolymphatic will demystify and simplify the subject somewhat as it helps in considering the venolymphatic malformations modes of clinical presentation, complications, and imaging appearance.
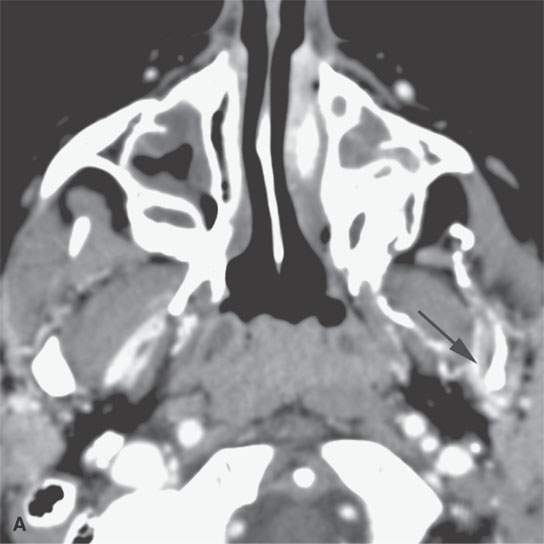
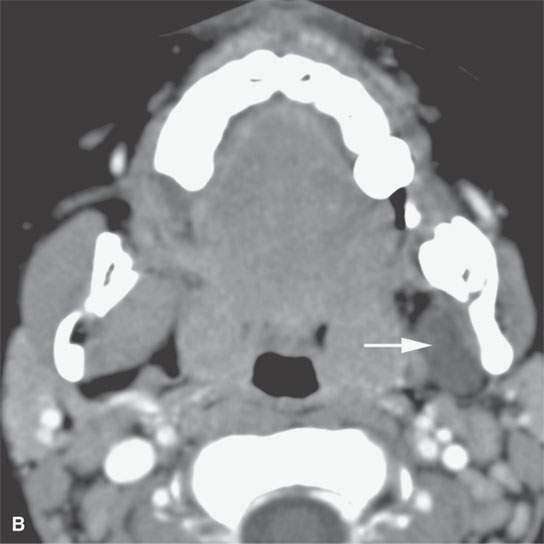
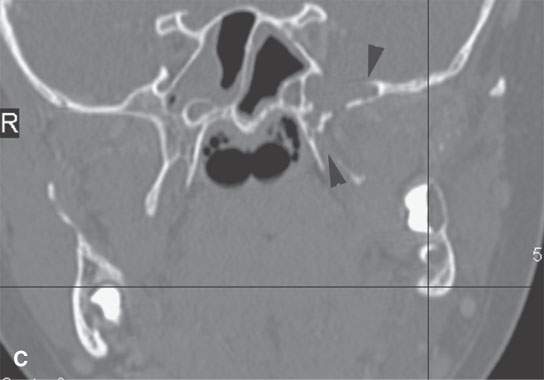
FIGURE 9.10. Contrast-enhanced computed tomography of a venolymphatic malformation with both blood-filled and lymph-filled spaces. A: Enhancing component communicating with the pterygoid venous plexus (arrow). B: Lymph-containing space (arrow). C: Component within the base of the sphenoid and base of the pterygoid plates (arrowheads) with potential intracranial communications, although none were specifically demonstrated.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree