- •
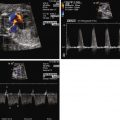
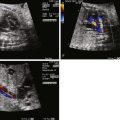
Lucency in the lung with color flow imaging highlighting increased flow within the lucency.
- •
Dilated pulmonary veins and selective dilation of the left side of the heart.
- •
Enlarged heart, but with relatively preserved systolic function.
- •
Reversal of flow in the ductus arteriosus with flow from aorta to pulmonary artery, but in the absence of any right-sided obstruction.
- •
Inequity in size and flow between the branch pulmonary arteries.
Anatomy and Anatomical Associations
Pulmonary arteriovenous fistula or malformation (PAVM) is a direct connection between the pulmonary arterial system and the pulmonary veins, bypassing the capillaries. Its effect is to deliver pulmonary arterial blood to the pulmonary veins and left side of the heart, bypassing the gas exchange unit of the lung. In the postnatal state, this results in deoxygenated blood reaching the systemic circulation. In the fetus, it can lead to heart failure, hydrops, and altered lung development, resulting in profound postnatal hypoxemia.
PAVMs are most typically seen in the adult patient with hereditary hemorrhagic telangiectasia (HHT), an autosomal dominant disorder in which multiple body telangiectasia are present. These vascular abnormalities may be present in the lung, liver, kidney, or head, causing symptoms ranging from epistaxis to organ hemorrhage and infarction. When present in the lung, the fistulous connections may allow for right-to-left shunting with paradoxical embolism.
PAVM is rare in childhood, and rarer still in the fetus. When seen early in life, they may be isolated and not necessarily associated with HHT. PAVM appears to be an acquired phenomenon in some patients with single-ventricle type of congenital heart disease who have undergone a cavopulmonary connection. When present in the fetus, PAVM is typically large and may adversely affect both heart and lung development.
Frequency, Genetics, and Development
PAVM in the fetus is extremely rare, with only a few case reports in the literature; however, it is a fascinating anomaly worthy of review and our attention. There are no known genetic associations beyond HHT, which is predominantly a disease that manifests in childhood with progression in adulthood. HHT does not typically manifest in the fetus.
There are interesting data to suggest that bypass connections between the pulmonary arterial system and the pulmonary venous system are normally present in the developing fetus. Animal work in the sheep using contrast echocardiography demonstrates significant right-to-left intrapulmonary shunting in the fetus, with persistence in the early newborn period, but disappearance at 4 weeks of life. Development of PAVMs later in life, for example, after cavopulmonary connection, may be a return to an earlier primitive stage of development.
Hepatic venous flow may play a role in the development of PAVM. It has been observed in various forms of cavopulmonary connection that, if hepatic effluent is not directed toward the lungs, such patients are at highest risk for development of PAVM. Furthermore, surgical connection and redirection of hepatic venous effluent into the lungs leads to resolution of PAVM. It is plausible to speculate that a similar phenomenon is at hand in the fetus. In the fetal circulation, hepatic venous and portal venous flow are markedly different than in the postnatal state. In addition, streaming patterns within the floor of the right atrium direct flow across the foramen ovale from right to left. It is conceivable that nature has created a natural pathway in which an “hepatic factor” is diverted away from the lungs during fetal life in order to allow for intentional intrapulmonary shunting through natural arterial-to-venous connections. With birth, the hepatic factor is redirected toward the lungs and these connections dissipate, except in unnatural cases such as various forms of congenital heart disease after palliation, in which the hepatic factor is once again denied entry into the lungs.
Prenatal Physiology
In the fetus, a large PAVM will act as a low vascular resistance “sink” and will drive blood to the region of the lung in which it is present. PAVM leads to an increased volume load, which may cause cardiac enlargement and lead to heart failure and hydrops. As blood is directed toward the PAVM, pulmonary venous return increases substantially because it is carrying both normal pulmonary venous effluent and the additional volume load that is being shunted into the PAVM. As a consequence, the pulmonary veins may become grossly enlarged and dilated and may appear as an echolucent region within the hilum of the lung. The volume load in PAVM is primarily carried by the left ventricle because it receives the increased pulmonary venous return. Left atrial pressure may be increased above normal due to torrential pulmonary venous return. Shunting at the foramen ovale may reverse from the normal right-to-left direction to left-to-right, as left atrial pressure exceeds right atrial pressure.
The low vascular sink of the PAVM may also drive blood into the lung via the ductus arteriosus. Blood flow in the fetal ductus arteriosus is normally directed from the pulmonary artery toward the descending aorta in an antegrade manner, diverting blood away from the normally high-resistance pulmonary vasculature. However, in the presence of a PAVM, the lung itself is now the pathway of least resistance and blood flow in the ductus arteriosus may be reversed, from the aorta into the main pulmonary artery, and further directed toward the lung and segments containing the PAVM. This can create a “steal” from the systemic circulation and further adds to the ventricular volume load.
Fetal PAVM can also inhibit normal lung development. The low vascular resistance sink of a PAVM will divert blood toward the region of the lung containing the PAVM and steal blood away from other regions. This creates a heterogeneous perfusion profile with some lung segments receiving excess flow and others receiving a paucity of flow. Such alterations in flow lead to histopathological changes in the pulmonary microvasculature. PAVM can, therefore, lead to secondary changes in the pulmonary vasculature that may further contribute to difficulties at birth, such as pulmonary hypertension, once placental separation takes place.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree