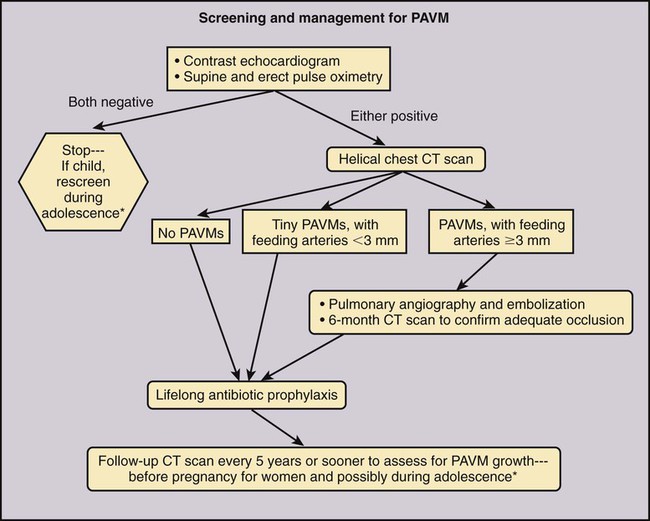
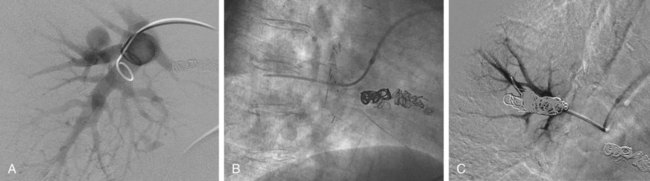
Jeffrey S. Pollak, Katharine Henderson and Robert I. White, Jr. Pulmonary arteriovenous malformations (PAVMs) are an important cause of stroke and transient ischemic attack (TIA), as well as brain abscess and other systemic infections due to loss of pulmonary capillary circulation filtering capacity.1 They may also spontaneously rupture and can be a cause of serious if not lethal hemoptysis and hemothorax, particularly in women during the third trimester of pregnancy and in children and adults with large PAVMs.2,3 Patients with large or multiple PAVMs often present with significant hypoxemia resulting in dyspnea and fatigue. A particularly difficult group to treat is a small set of patients with diffuse pulmonary malformations, a category less well recognized.4,5 Hereditary hemorrhagic telangiectasia (HHT), the underlying genetic disorder in most patients with PAVM, is common among rare diseases (200 per million people), and of these patients, approximately a third will have PAVM. Worldwide centers now exist for management of PAVM and the other organ manifestations of HHT (www.hht.org). Screening of affected families for PAVM is most easily done by contrast echocardiography. If positive, noncontrast thin-section computed tomography (CT) of the chest is done to size the PAVM (Fig. 85-1).6,7 Screening for PAVM should be done periodically in affected children by pulse oximetry and, after age 12, by contrast echocardiography.3 Large PAVMs in children younger than 12 years of age should be treated because of the risk of pulmonary hemorrhage.3 In adolescents and adults, all PAVMs with arteries 3 mm or greater in diameter should be treated.8,9 Recently we developed an algorithm for screening and management of children under 12. Based on our experience at the Yale HHT Center, neither embolic stroke due to paradoxical embolus nor brain abscess have occurred in the children we have examined who had standing oxygen saturation greater than 97%. In a recent study,10 we found exercise stress testing to be a highly reproducible test in children and adults with PAVM. Although not yet accepted by others, it is our hope that the use of exercise stress testing in patients with HHT will replace the perceived need for early contrast echocardiography and excessive use of CT.10 This is important because early treatment of small PAVMs in children leads to collateral reperfusion, irrespective of the device used to close the PAVM. Collateral reperfusion requires additional treatment and radiation exposure at a vulnerable age.10 Although there are no absolute contraindications for embolotherapy of PAVM, there are some aspects of the closure technique that require special attention. First, in patients with secondary or primary pulmonary hypertension, care should be taken so that PAVM closure does not raise pulmonary artery pressures to a level that poses a risk of right-sided heart failure. Measurement of pulmonary artery pressures is essential before occluding PAVMs. Patients with symptomatic liver malformations will have moderate elevations in pulmonary artery pressure (40-60/20-30 mmHg). Also, there is an association between HHT and primary pulmonary hypertension.11 Second, in patients with high-flow arteries less than 2.5 cm in length, closure of the sac connecting the artery to the vein should be considered, and special techniques are required.12 Sac closure with oversized or detachable coils is expensive and time consuming, and at our institution was necessary only 6 times in more than 1000 consecutive patients. Recently, with the introduction and short-term experience with the detachable HydroCoil system (Terumo Medical Corp., Somerset, N.J.) and the Amplatzer II Vascular Plug (St. Jude Medical, St. Paul, Minn.), we believe sac closure will be needed much less often. Our standard techniques have been outlined in recent articles.9,13 We feel that pushable coils suffice for treating most PAVMs, although detachable coils like the AZUR HydroCoil (Terumo), which is fully retractable before final deployment, as well as the Amplatzer plug, will have a role in managing some PAVMs (Fig. 85-2). Interventional radiologists treating PAVMs should have familiarity with both of these techniques and the standard techniques described.14,15 Standard equipment used for 90% of PAVMs includes: • A 7F sheath placed in either femoral vein and connected to heparinized saline after careful exclusion of all air bubbles • A 5F pigtail catheter for diagnostic pulmonary angiography • A 7/5F coaxial LuMax (Cook Medical, Bloomington, Ind.) guide catheter is exchanged for the pigtail, using a reinforced exchange wire. The 100-cm, 5F angled end-hole catheter that comes with the set is used for most occlusions. The 80-cm, 7F guide catheter is placed just proximal to the 5F catheter and provides stability for deployment of tightly packed coils either by the “anchor” or “scaffold” technique (see Anatomy and Approaches). • The same coaxial catheter approaches apply when using a detachable coil; Amplatzer II plugs up to 12 mm can be placed through the guiding catheter. For patients with short high-flow arteries (i.e., < 2.5 cm long), embolization is performed through the occlusion balloon catheter (Boston Scientific) with a double-marker Renegade 0.021-inch microcatheter (Boston Scientific) and deployment-suitable pushable, and perhaps detachable, 0.018-inch coils. The resultant coil mass should be at least twice the diameter of the draining pulmonary veins and should fill the aneurysmal sac. Once the sac is filled with oversized microcoils, the occlusion balloon catheter is withdrawn and the occlusion completed with the 7/5F LuMax system and standard 0.035- or 0.038-inch fibered coils.12 Most PAVMs consist of a variable-length single segmental artery, but 10% are complex, with more than one segmental artery connecting the aneurysmal sac to the draining veins.9,16,17 Despite the magnificent images provided by high-resolution multidetector CT scanners, as well as similar images from magnetic resonance angiography (MRA), we still depend on multiprojection diagnostic pulmonary angiography before embolotherapy. It is particularly important to be confident of the morphology of the artery as it enters the sac. The goal of therapy is to occlude the feeding artery as close to the sac as possible, thereby limiting reperfusion from adjacent branches in the lungs of children3 and limiting reperfusion from the bronchial circulation, which is known to occur with proximal occlusions.18,19
Pulmonary Arteriovenous Malformations
Diagnosis and Management
Indications
Contraindications
Equipment
Specialized Equipment for Other Anatomy and Physiology
Technique
Anatomy and Approaches
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Radiology Key
Fastest Radiology Insight Engine