Schemes for Staging
The most widely used scheme for staging non–small cell lung cancer (NSCLC) is the TNM classification. A variety of alterations in this scheme have been made to better group patients with similar prognosis and treatment options. The seventh edition of the TNM classification of lung cancer, published in 2009, was based on the analysis of a retrospective international database collected from 1990 to 2000 by the International Association for the Study of Lung Cancer (IASLC) ( Table 18.1 ). The revision in the seventh edition consisted of changes in the T descriptors that emphasized the prognostic impact of tumor size and redefined the classification of additional tumor nodules and malignant pleural effusion, the subclassification of M1, the validation of the classification for bronchopulmonary carcinoid tumors, and the rearrangement of stage grouping, whereas the N descriptors remained the same. Recently, the IASLC had collected new data of 77,156 patients diagnosed with lung cancer from 1999 to 2010. This new database was used to inform the eighth edition of the TNM classification for lung cancer, due to be published in 2018. In this chapter, all descriptors for T, N, M and TNM stage groups of NSCLC and staging of small cell lung cancer (SCLC) will be based on the eighth edition of the TNM classification for lung cancer. Specific properties of each of the T, N, and M subtypes as proposed by this revision are shown in Table 18.2 . The various combinations of T, N, and M that define different stages are depicted in Table 18.3 .
- •
T (tumor), N (node), and M (metastasis) (TNM) system is used for determining tumor subgrouping and staging for lung cancer.
- •
The eighth edition of the TNM Classification for Lung Cancer was proposed by the International Association for the Study of Lung Cancer (IASLC).
| TX | Primary tumor cannot be assessed, or tumor proven by the presence of malignant cells in sputum or bronchial washings but not visualized by imaging or bronchoscopy |
| T0 | No evidence of primary tumor |
| Tis | Carcinoma in situ |
| T1 | Tumor ≤ 3 cm in greatest dimension, surrounded by lung or visceral pleura, without bronchoscopic evidence of invasion more proximal than the lobar bronchus a (i.e., not in the main bronchus) |
| T1a: tumor ≤ 2 cm in greatest dimension | |
| T1b: tumor > 2 cm, ≤ 3 cm in greatest dimensions | |
| T2 | Tumor > 3 cm, ≤ 7 cm; or tumor with any of the following features: involves main bronchus, ≥ 2 cm distal to the carina, invades the visceral pleura, associated with atelectasis or obstructive pneumonitis that extends to the hilar region but does not involve the entire lung |
| T2a: tumor > 3 cm, ≤ 5 cm in greatest dimension | |
| T2b: tumor > 5 cm, ≤ 7 cm in greatest dimension | |
| T3 | Tumor > 7 cm or any size that directly invades any of the following: chest wall (including superior sulcus tumor), diaphragm, phrenic nerve, mediastinal pleura, parietal pericardium; or tumor in the main bronchus < 2 cm distal to the carina but without involvement of the carina; or associated atelectasis or obstructive pneumonitis of the entire lung or separate tumor nodule(s) in the same lobe as the primary |
| T4 | Tumor of any size that invades any of the following: mediastinum, heart, great vessels, trachea, recurrent laryngeal nerve, esophagus, vertebral body, carina; separate tumor nodule(s) in a different ipsilateral lobe to that of the primary |
| NX | Regional lymph node cannot be assessed |
| N0 | No regional lymph node metastasis |
| N1 | Metastasis to ipsilateral peribronchial and/or ipsilateral hilar lymph nodes, and intrapulmonary nodes involved by direct extension of the primary tumor |
| N2 | Metastasis to ipsilateral mediastinal and/or subcarinal lymph node(s) |
| N3 | Metastasis to contralateral mediastinal, contralateral hilar, ipsilateral or contralateral scalene, or supraclavicular lymph node(s) |
| MX | Presence of distant metastasis cannot be assessed |
| M0 | No distant metastasis |
| M1 | Distant metastasis present |
| M1a: separate tumor nodule(s) in a contralateral lobe; tumor with pleural nodules or malignant pleural or pericardial effusion b | |
| M1b: distant metastasis |
a The uncommon superficial tumor of any size with its invasive component limited to the bronchial wall, which may extend proximal to the main bronchus, is also classified as T1a.
b Most pleural (pericardial) effusions with lung cancer are due to tumor. In a few patients, however, multiple microscopic examinations of pleural (pericardial) fluid are negative for tumor, and the fluid is nonbloody and is not an exudate. When these elements and clinical judgment dictate that the effusion is not related to the tumor, the effusion should be excluded as a staging element, and the patient should be classified as M0.
| TX | Primary tumor cannot be assessed, or tumor proven by the presence of malignant cells in sputum or bronchial washings but not visualized by imaging or bronchoscopy |
| T0 | No evidence of primary tumor |
| Tis | Carcinoma in situ |
| T1 | Tumor ≤ 3 cm in greatest dimension, surrounded by lung or visceral pleura, without bronchoscopic evidence of invasion more proximal than the lobar bronchus a (i.e., not in the main bronchus) |
| T1a(mi): minimally invasive adenocarcinoma b | |
| T1a: tumor ≤ 1 cm in greatest dimension | |
| T1b: tumor > 1 cm, ≤ 2 cm in greatest dimension | |
| T1c: tumor > 2 cm, ≤ 3 cm in greatest dimension | |
| T2 | Tumor > 3 cm, ≤ 5 cm; or tumor with any of the following features: involves main bronchus regardless of distance from the carina without involvement of the carina, invades the visceral pleura, associated with atelectasis or obstructive pneumonitis |
| T2a: tumor > 3 cm, ≤ 4 cm in greatest dimension | |
| T2b: tumor > 4 cm, ≤ 5 cm in greatest dimension | |
| T3 | Tumor > 5 cm, ≤ 7 cm in greatest dimension; or directly invades any of the following: chest wall (including parietal pleura and superior sulcus tumor), phrenic nerve, parietal pericardium; separate tumor nodule(s) in the same lobe as the primary |
| T4 | Tumor > 7 cm in greatest dimension or associated with separate tumor nodule(s) in a different ipsilateral lobe to that of the primary or direct invasion of any of the following: diaphragm, mediastinum, heart, great vessels, trachea, recurrent laryngeal nerve, esophagus, vertebral body, carina |
| NX | Regional lymph node cannot be assessed |
| N0 | No regional lymph node metastasis |
| N1 | Metastasis to ipsilateral peribronchial and/or ipsilateral hilar lymph nodes and intrapulmonary nodes involved by direct extension of the primary tumor |
| N2 | Metastasis to ipsilateral mediastinal and/or subcarinal lymph node(s) |
| N3 | Metastasis to contralateral mediastinal, contralateral hilar, ipsilateral or contralateral scalene, or supraclavicular lymph node(s) |
| MX | Presence of distant metastasis cannot be assessed |
| M0 | No distant metastasis |
| M1 | Distant metastasis present |
| M1a: separate tumor nodule(s) in a contralateral lobe; tumor with pleural nodules or malignant pleural or pericardial effusion c | |
| M1b: single extrathoracic metastasis d | |
| M1c: multiple extrathoracic metastases in one or more organs |
a The uncommon superficial tumor of any size with its invasive component limited to the bronchial wall, which may extend proximal to the main bronchus, is also classified as T1a.
b Solitary adenocarcinoma, ≤3 cm with a predominantly lepidic pattern and ≤5-mm invasion in any one focus.
c Most pleural (pericardial) effusions with lung cancer are due to tumor. In a few patients, however, multiple microscopical examinations of pleural (pericardial) fluid are negative for tumor, and the fluid is nonbloody and is not an exudate. When these elements and clinical judgment dictate that the effusion is not related to the tumor, the effusion should be excluded as a staging element, and the patient should be classified as M0.
d This includes involvement of a single distant (nonregional) lymph node.
| Stage | TNM Subset |
|---|---|
| Occult carcinoma | TXN0M0 |
| 0 | TisN0M0 |
| IA1 | T1a(mi)N0M0 T1aN0M0 |
| IA2 | T1bN0M0 |
| IA3 | T1cN0M0 |
| IB | T2aN0M0 |
| IIA | T2bN0M0 |
| IIB | T1a-cN1M0 T2aN1M0 T2bN1M0 T3N0M0 |
| IIIA | T1a-cN2M0 T2a-bN2M0 T3N1M0 T4N0M0 T4N1M0 |
| IIIB | T1a-cN3M0 T2a-bN3M0 T3N2M0 T4N2M0 |
| IIIC | T3N3M0 T4N3M0 |
| IVA | Any T, Any N M1a Any T, Any N M1b |
| IVB | Any T, Any N M1c |
Methods of Staging
A variety of techniques can be used to investigate T, N, and M parameters to determine the appropriate tumor stage.
T (Primary Tumor)
Radiography
The presence of pulmonary carcinoma is often first suspected on the chest radiograph. The radiograph also provides information about the T staging by demonstrating the size of the lesion in patients in whom it is circumscribed and the degree of associated atelectasis or obstructive pneumonitis in the presence of airway obstruction in patients in whom it is not circumscribed. It may also show the presence of pleural effusion and, in some cases, evidence of chest wall or mediastinal invasion. Direct extension of a neoplasm into the chest wall may be established by radiographic evidence of destruction of ribs or vertebrae or clinical evidence of a palpable mass. Evidence of invasion into the mediastinum may be suggested by marked elevation of a hemidiaphragm (related to phrenic nerve paralysis). With these few exceptions, however, the chest radiograph is unreliable in detecting invasion of the chest wall, diaphragm, or the mediastinum.
Computed Tomography
The seventh edition of the TNM classification for lung cancer emphasized the prognostic impact of the tumor size and subclassified T descriptors according to the tumor size. This finding was also confirmed in the new IASLC database for the eighth edition of the TNM classification. Although the 3-cm cutpoint still remains a landmark to separate T1 from T2 tumors, the survival analyses showed that a progressive degradation of survival was observed for each 1-cm cutpoint. On the basis of the new IASLC database, the following rearrangement of T descriptors was provided: T1 tumors were subdivided into three subgroups at 1-cm cutpoints; T2 tumors were subdivided into two subgroups; T2 tumors greater than 5 cm and less than or equal to 7 cm were reclassified as T3; T3 tumors greater than 7 cm were reclassified as T4 (see Table 18.2 ). The survival analysis also showed that involvement of the main bronchus either less than 2 cm or more than 2 cm from the carina has a similar prognosis. Therefore the new IASLC database provided that T3 tumors classified by endobronchial location were combined as T2 tumor. Total atelectasis or pneumonitis, a T3 descriptor in the seventh edition, showed better prognosis than other T3 tumors in the new IASLC database; therefore the IASLC project provided grouping of partial and total atelectasis/pneumonitis as a T2 descriptor. It also reclassified diaphragm invasion as a T4 descriptor. Mediastinal pleural invasion without mediastinal tissue invasion is difficult to determine clinically and is rare at pathologic staging; therefore the new IASLC staging deleted mediastinal pleural invasion as a T descriptor.
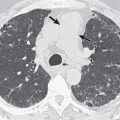
Other T3 and T4 descriptors were not changed from the seventh edition of TNM staging. It is important to emphasize that if a tumor abuts a pleural surface or when pleural thickening is noted immediately adjacent to a tumor mass, the CT findings must be interpreted as indeterminate or merely as suspicious for pleural and chest wall invasion. Chest wall invasion can be diagnosed confidently only when tumor obliterates the fat planes between parietal pleura and chest wall muscle or when there is associated bone destruction ( Figs. 18.1 and 18.2 ). Lung carcinomas are likely to be extensively invasive and unresectable (T4) if they involve the tracheal carina or surround, encase, or abut more than 180 degrees of the circumference of the aorta ( Fig. 18.3 ), main or proximal portion of the right or left pulmonary arteries, or the esophagus. Primary pulmonary carcinoma may be unresectable (T4) if it invades the heart, great vessels, or the vertebral body.



Magnetic Resonance Imaging
MRI is superior to CT in the demonstration of the pericardium, cardiac chambers, and mediastinal vessels with the added advantage of not requiring IV contrast medium (see Fig. 18.3 ). Disruption of the normal 2- to 3-mm-thickness low signal intensity of the pericardium is suggestive of pericardial infiltration, although this does not preclude complete surgical resection. Coronal images are particularly helpful in the assessment of tumor extension into the subcarinal region, aortopulmonary window, and superior vena cava.
MRI may allow better delineation of mediastinal and superior sulcus invasion ( Fig. 18.4 ). MRI is especially useful in the evaluation of brachial plexus, subclavian vessel, or vertebral body invasion in Pancoast tumors. However, there is no significant difference in the overall diagnostic accuracy between CT and MRI. In one large study, the sensitivity of CT and MRI was 63% and 56%, respectively, and the specificity was 84% and 80% for distinguishing T3 and T4 tumors from less extensive pulmonary carcinomas. Similar to CT, the main limitation of MRI is the inability to distinguish tumor invasion of mediastinal fat from inflammatory changes.

Disadvantages of MRI include lower spatial resolution than CT, longer scan time, higher cost, and artifacts as a result of respiratory motion. Recently introduced MRI techniques using fast (T2W half-Fourier acquisition single-shot turbo-spin-echo (HASTE), and T1W fat-saturated three-dimensional gradient-echo sequences with nearly isotropic resolution) and high-quality (breath-hold, electrocardiogram-gated, black-blood techniques on T2W turbo-spin-echo [TSE] and short-T1 inversion recovery [STIR] sequences) scan parameters have greatly improved the image quality and the potential role of MRI in the staging of lung cancer ( Figs. 18.5 and 18.6 ). Moreover, increased spatial resolution can be obtained using parallel acquisition and reconstruction techniques: sensitivity encoding (SENSE) and simultaneous acquisition of spatial harmonics (SMASH). Recent analyses of T staging using advanced MRI protocols showed that diagnostic accuracies of MRI were 82% to 94.3%, which were comparable to those of PET-CT (86%–91.4%).


Positron Emission Tomography
Integrated PET-CT provides morphologic as well as metabolic data of lung cancer and is widely accepted to be the first-line imaging tool for staging. It has been shown to be more useful than CT alone in determining the T stage of the primary tumor and in assessing chest wall invasion. According to a report, although statistically not significant ( P =.25), integrated PET-CT accurately staged the primary tumor (T stage) in 86% (91 of 106) of patients, whereas CT accurately staged the primary tumor in only 79% (84 of 106) of patients.
The main limitation of PET-CT in the T staging is false positivity in cases of inflammatory lesions. In this context, the newly introduced PET-MRI system with superior soft tissue contrast and dedicated sequences has the potential to compensate the shortcomings of PET-CT. According to the studies with comparison of PET-MRI and PET-CT in the preoperative staging of NSCLC, the diagnostic accuracy of PET-MRI (65%–94.3%) in the T staging was comparable to that of PET-CT (70%–91.4%).
- •
Chest radiography is generally unreliable in detecting invasion of the chest wall, diaphragm, or mediastinum.
- •
Computed tomography (CT) can reliably detect invasion of the mediastinum, provided that major mediastinal vessels or bronchi are surrounded by tumor.
- •
Magnetic resonance imaging (MRI) is superior to CT in the demonstration of the pericardium, cardiac chambers, and mediastinal vessels, with the added advantage of not requiring intravenous (IV) contrast medium. Recent analyses of T staging using advanced MRI protocols showed that diagnostic accuracies of MRI were 82%–94.3%, which were comparable to those of positron emission tomography (PET)-CT (86%–91.4%).
N (Lymph Nodes)
Radiography and Computed Tomography
Lymph node involvement in lung cancer is categorized according to the location of the metastatic lymph nodes as N0 (no nodes involved), N1 (ipsilateral peribronchial, interlobar, or hilar node involvement), N2 (ipsilateral mediastinal or subcarinal node involvement), or N3 (contralateral mediastinal, contralateral hilar, or supraclavicular node involvement), regardless of the number of involved lymph nodes. Analysis of the new IASLC database with respect to the N staging has shown that the N categories in the seventh edition of TNM staging for lung cancer are still useful for distinguishing among tumors with significantly different prognoses in both clinical and pathologic staging. However, with regard to pathologic staging, the survival curves for N1 at multiple stations and N2 at a single station with N1 involvement overlapped each other, and N2 at a single station without N1 involvement had a better prognosis than N1 at multiple stations, although the difference was not significant. The following represents the most widely accepted criteria for radiologic assessment.
- 1.
Lymph nodes should be classified according to a standardized lymph node map. The one adopted by the American Joint Committee on Cancer and the International Union Against Cancer in 2009 ( Fig. 18.7 and Table 18.4 ) is the IASLC nodal map and anatomic definitions, and it is still the recommended means of describing regional lymph node involvement for lung cancers in the proposed eighth edition.

Fig. 18.7
The International Association for the Study of Lung Cancer (IASLC) lymph node map, including the grouping of lymph node stations into “zones” for the purpose of prognostic analyses. Ao, Aorta; AP, aortopulmonary zone; Eso, esophagus; mPA, main pulmonary artery; SVC, superior vena cava; T, trachea.
(Reprinted with permission from the International Association for the Study of Lung Cancer. The IASLC Lung Cancer Project. A proposal for a new international lymph node map in the forthcoming seventh edition of the TNM classification for lung cancer. J Thoracic Oncol . 2009;4:568–577.)
TABLE 18.4
LYMPH NODE MAP DESCRIPTION
Nodal Station
Anatomic Landmarks
- 1.
Low cervical, supraclavicular and sternal notch nodes
Upper border: lower margin of cricoid cartilage; lower border: clavicles bilaterally and, in the midline, the upper border of the manubrium
The oncologic midline is the midline of the trachea.
- 2.
Upper paratracheal nodes
2R and 2L: upper border: apex of lung and pleural space and, in the midline, the upper border of the manubrium; 2R lower border: intersection of caudal margin of innominate vein with the trachea
2L lower border: superior border of the aortic arch (the oncologic midline is along the left lateral border of the trachea)
- 3.
Prevascular and retrotracheal nodes
3a: prevascular
Right: upper border: apex of chest; lower border: level of carina; anterior border: posterior aspect of sternum; posterior border: anterior border of superior vena cava
Left: upper border: apex of chest; lower border : level of carina; anterior border: posterior aspect of sternum; posterior border: left carotid artery
3p: retrotracheal
Upper border: apex of chest; lower border: carina; anterior border: posterior tracheal wall
- 4.
Lower paratracheal nodes
4R: includes right paratracheal nodes, and pretracheal nodes extending to the left lateral border of trachea
Upper border: intersection of caudal margin of innominate vein with the trachea; lower border: lower border of azygos vein
4L: includes nodes to the left of the left lateral border of the trachea, medial to the ligamentum arteriosum
Upper border: upper margin of the aortic arch; lower border: upper rim of the left pulmonary artery
- 5.
Subaortic (aortopulmonary window)
Subaortic nodes lateral to the ligamentum arteriosum
Upper border: the lower border of the aortic arch; lower border: upper rim of the left pulmonary artery
- 6.
Paraaortic nodes (ascending aorta or phrenic)
Nodes lying anterior and lateral to the ascending aorta and the aortic arch
Upper border: a line tangential to the upper border of the aortic arch; lower border: the lower border of the aortic arch
- 7.
Subcarinal nodes
Upper border: the carina of the trachea; lower border: the upper border of the lower lobe bronchus on the left; the lower border of the bronchus intermedius on the right
- 8.
Paraesophageal nodes (below carina)
Nodes lying adjacent to the wall of the esophagus and to the right or left of the midline, excluding subcarinal nodes
Upper border: the upper border of the lower lobe bronchus on the left; the lower border of the bronchus intermedius on the right; lower border: the diaphragm
- 9.
Pulmonary ligament nodes
Nodes lying within the pulmonary ligament
Upper border: the inferior pulmonary vein; lower border: the diaphragm
- 10.
Hilar nodes
Includes nodes immediately adjacent to the mainstem bronchus and hilar vessels including the proximal portions of the pulmonary veins and main pulmonary artery
Upper border: the lower rim of the azygos vein on the right; upper rim of the pulmonary artery on the left
Lower border: interlobar region bilaterally
- 11.
Interlobar nodes
Between the origin of the lobar bronchi
- 12.
Lobar nodes
Nodes lying adjacent to the lobar bronchi
- 13.
Segmental nodes
Nodes lying adjacent to the segmental bronchi
- 14.
Subsegmental nodes
Nodes lying adjacent to the subsegmental bronchi 
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


- 1.