• A pulmonary embolus (PE) usually arises from a thrombus within a pelvic or lower limb vein and normally lodges within the branch pulmonary arteries • Risk factors for a pulmonary embolism: increasing age • d-Dimers: these are a breakdown product of cross-linked fibrin (and therefore a measure of fibrinolytic activity) • This has a low sensitivity and specificity – however it may exclude other causes (e.g. a pneumothorax) • The most common signs (without infarction): • Signs associated with infarction: • CTPA is a sensitive method of detecting main, lobar and segmental pulmonary arterial emboli – ‘Tram track’ appearance: contrast medium flows around or is adjacent to a clot if the vessel is within the image plane • This assesses the distribution of the pulmonary blood flow This assesses the distribution of the inhaled air • Technique: this is performed by the inhalation of krypton-81m, xenon-133, 99mTc-DTPA or ‘technegas’ • 81mKr: this is the optimal imaging agent, emitting high-energy photons (190keV) • 133Xe: although it is cheaper than 81mKr, it is a less optimal imaging agent owing to its longer half-life (5.3 days) and low photon energies (80keV) • 99mTc-DTPA and technegas aerosols: these are administered via a nebulizer during inspiration
Pulmonary circulation and thromboembolism
PULMONARY THROMBOEMBOLIC DISEASE
PULMONARY THROMBOEMBOLIC DISEASE
DEFINITION
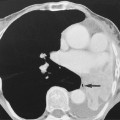
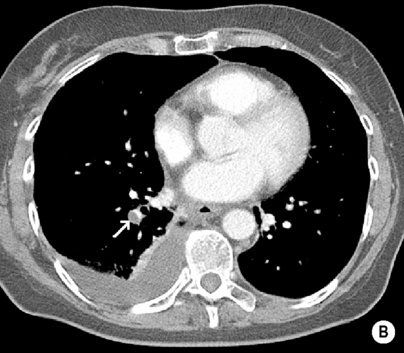
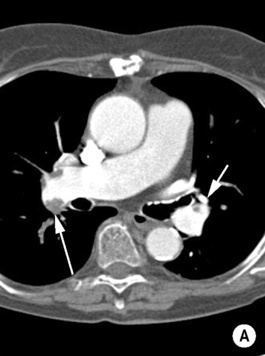
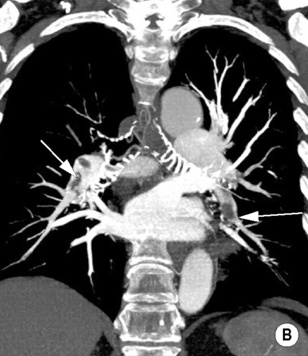
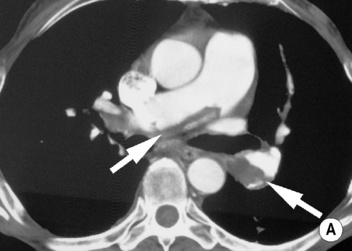
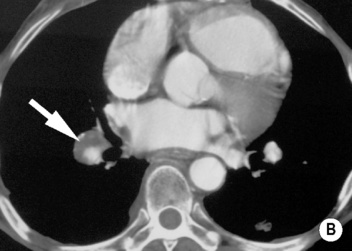
 ‘Saddle’ embolus: a thrombus lodged at the main pulmonary arterial bifurcation
‘Saddle’ embolus: a thrombus lodged at the main pulmonary arterial bifurcation
 Pulmonary infarction: This is relatively rare as there is a second ‘systemic’ arterial supply to the lungs from the bronchial arteries – infarction therefore requires compromise of both arterial supplies
Pulmonary infarction: This is relatively rare as there is a second ‘systemic’ arterial supply to the lungs from the bronchial arteries – infarction therefore requires compromise of both arterial supplies
 hypercoagulable state
hypercoagulable state  orthopaedic surgery
orthopaedic surgery  immobilization
immobilization  malignancy
malignancy  medical illness
medical illness  pregnancy
pregnancy  oestrogen use
oestrogen use
 it is a highly sensitive but non-specific test (with a high false-positive rate but a very high negative predictive value)
it is a highly sensitive but non-specific test (with a high false-positive rate but a very high negative predictive value)  false-negative tests can occur (particularly with subsegmental emboli)
false-negative tests can occur (particularly with subsegmental emboli)
RADIOLOGICAL FEATURES
CXR
 Westermark’s sign: localized peripheral oligaemia secondary to an embolus lodging in a peripheral artery
Westermark’s sign: localized peripheral oligaemia secondary to an embolus lodging in a peripheral artery  this may be associated with proximal arterial dilatation
this may be associated with proximal arterial dilatation
 Peripheral airspace opacification: this represents pulmonary haemorrhage
Peripheral airspace opacification: this represents pulmonary haemorrhage
 Linear atelectasis: ischaemic injury to type II pneumocytes leads to surfactant deficiency
Linear atelectasis: ischaemic injury to type II pneumocytes leads to surfactant deficiency
 Pleural effusions: these are often small
Pleural effusions: these are often small
 Central pulmonary arterial enlargement: this is secondary to chronic repeated embolic disease
Central pulmonary arterial enlargement: this is secondary to chronic repeated embolic disease
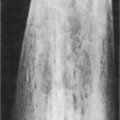
 Hampton’s hump: a pleurally based, wedge-shaped opacity normally seen within the lateral or posterior costophrenic sulcus
Hampton’s hump: a pleurally based, wedge-shaped opacity normally seen within the lateral or posterior costophrenic sulcus  the apex of the triangle points toward the occluded feeding vessel with its base against the pleural surface
the apex of the triangle points toward the occluded feeding vessel with its base against the pleural surface  it rarely contains air bronchograms
it rarely contains air bronchograms  it is a rare and non-specific sign
it is a rare and non-specific sign
 Consolidation (which may be multifocal): this predominantly affects the lower lobes
Consolidation (which may be multifocal): this predominantly affects the lower lobes  it can be seen from 12 h to several days post embolism
it can be seen from 12 h to several days post embolism
 Cavitation: this follows secondary infection at the infarction site or following a septic embolus
Cavitation: this follows secondary infection at the infarction site or following a septic embolus
 Haemorrhagic pleural effusions: this is seen in 50% of patients
Haemorrhagic pleural effusions: this is seen in 50% of patients
 Serial CXRs: rapid resolution of any parenchymal changes is associated with a non-infarcting PE – infarction normally heals with scarring and localized pleural thickening
Serial CXRs: rapid resolution of any parenchymal changes is associated with a non-infarcting PE – infarction normally heals with scarring and localized pleural thickening
Computed tomography pulmonary angiography (CTPA)
 The imaged area encompasses the central and segmental pulmonary arteries (i.e. from the aortic arch to the inferior pulmonary veins)
The imaged area encompasses the central and segmental pulmonary arteries (i.e. from the aortic arch to the inferior pulmonary veins)
 Contrast medium concentrations > 120–250mg/ml can be associated with significant streak artefacts (particularly adjacent to the SVC)
Contrast medium concentrations > 120–250mg/ml can be associated with significant streak artefacts (particularly adjacent to the SVC)
 Accurate timing of data acquisition (post IV contrast administration) is essential in order to achieve optimum pulmonary arterial opacification – data is usually only acquired when a predefined level of pulmonary arterial enhancement is achieved (this is detected by repeated imaging over a chosen region of interest)
Accurate timing of data acquisition (post IV contrast administration) is essential in order to achieve optimum pulmonary arterial opacification – data is usually only acquired when a predefined level of pulmonary arterial enhancement is achieved (this is detected by repeated imaging over a chosen region of interest)
 Contrast density < 200–250HU usually implies a non-diagnostic study
Contrast density < 200–250HU usually implies a non-diagnostic study
 it may reliably detect emboli in up to 4th-order vessels (which are 7mm in diameter)
it may reliably detect emboli in up to 4th-order vessels (which are 7mm in diameter)
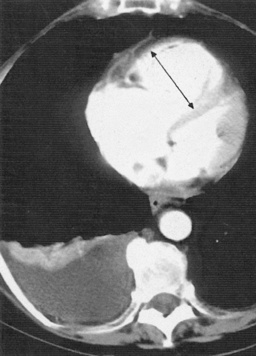
 Acute emboli: an intravascular filling defect
Acute emboli: an intravascular filling defect  it may appear as an expanded unopacified vessel
it may appear as an expanded unopacified vessel
 Chronic emboli: a crescentic thrombus adherent to the arterial wall (which may also be calcified or show evidence of recanalization)
Chronic emboli: a crescentic thrombus adherent to the arterial wall (which may also be calcified or show evidence of recanalization)  enlargement of the bronchial vessels (representing the collateral supply)
enlargement of the bronchial vessels (representing the collateral supply)  a prominent mosaic attenuation pattern
a prominent mosaic attenuation pattern
 Infarction: a peripheral wedge-shaped region of consolidation (analogous to a Hampton’s hump on CXR)
Infarction: a peripheral wedge-shaped region of consolidation (analogous to a Hampton’s hump on CXR)  this is only a specific sign if the vessel can be traced to the apex of the wedge
this is only a specific sign if the vessel can be traced to the apex of the wedge
PULMONARY THROMBOEMBOLIC DISEASE
PERFUSION (Q) SCINTIGRAPHY
 particles microembolize within the lung, providing a map of the pulmonary blood flow (only approximately 2% of the capillaries are occluded)
particles microembolize within the lung, providing a map of the pulmonary blood flow (only approximately 2% of the capillaries are occluded)  the effective dose is < 1mSv
the effective dose is < 1mSv
VENTILATION (V) SCINTIGRAPHY
 8 images are conventionally acquired (anterior, posterior, oblique and lateral projections on both sides)
8 images are conventionally acquired (anterior, posterior, oblique and lateral projections on both sides)
 its higher photon energy allows the ventilation images to be obtained after the perfusion images as well as allowing matching of both image sets without moving the patient
its higher photon energy allows the ventilation images to be obtained after the perfusion images as well as allowing matching of both image sets without moving the patient  owing to its short half-life (13 s) it can be continuously administered (including during perfusion imaging) although it means that no washout images are possible
owing to its short half-life (13 s) it can be continuously administered (including during perfusion imaging) although it means that no washout images are possible
 Disadvantages: there is decreased resolution due to collimator penetration by the high-energy photons
Disadvantages: there is decreased resolution due to collimator penetration by the high-energy photons  it is expensive
it is expensive
 ventilation studies need to be performed prior to any perfusion studies (thus preventing Compton scatter from the 99mTc into the lower 133Xe photopeak)
ventilation studies need to be performed prior to any perfusion studies (thus preventing Compton scatter from the 99mTc into the lower 133Xe photopeak)
 Single breath inhalation image: a cold spot is abnormal
Single breath inhalation image: a cold spot is abnormal
 Equilibirum phase: tracer activity corresponds to aerated lung
Equilibirum phase: tracer activity corresponds to aerated lung
 Washout phase: tracer retention corresponds to areas of air trapping (e.g. COPD)
Washout phase: tracer retention corresponds to areas of air trapping (e.g. COPD)
 the aerosol imaging provides a non-physiological static image of lung ventilation with central airway deposition demonstrated (81mKr allows dynamic imaging)
the aerosol imaging provides a non-physiological static image of lung ventilation with central airway deposition demonstrated (81mKr allows dynamic imaging)  technegas aerosols and 81mKr both provide better images than DTPA
technegas aerosols and 81mKr both provide better images than DTPA
 Disadvantage: it cannot be administered during perfusion (as both aerosols and MAA are labelled with 99mTc)
Disadvantage: it cannot be administered during perfusion (as both aerosols and MAA are labelled with 99mTc)
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Pulmonary circulation and thromboembolism

 chest pain (which may be pleuritic)
chest pain (which may be pleuritic)  haemoptysis
haemoptysis  cough
cough  hypotension
hypotension  tachycardia
tachycardia  pulmonary oedema (due to left ventricular failure precipitated by a large PE)
pulmonary oedema (due to left ventricular failure precipitated by a large PE)
 right bundle branch block
right bundle branch block  right axis deviation and ventricular hypertrophy
right axis deviation and ventricular hypertrophy persistent intracardiac shunts
persistent intracardiac shunts  pregnancy (the increased cardiac output may result in the majority of the contrast residing beyond the pulmonary arteries when imaging)
pregnancy (the increased cardiac output may result in the majority of the contrast residing beyond the pulmonary arteries when imaging)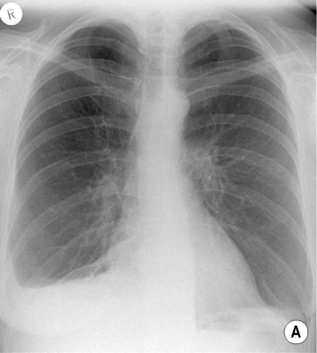

 the preparation should not be ‘backflushed’ with blood in order to prevent coagulation and ‘hot spots’ forming on the lungs
the preparation should not be ‘backflushed’ with blood in order to prevent coagulation and ‘hot spots’ forming on the lungs