1. Pulmonary arterial hypertension
1.1 Idiopathic PAH
1.2 Heritable PAH
1.2.1 BMPR2
1.2.2 ALK-1, ENG, SMAD9, CAV1, KCNK3
1.2.3 Unknown
1.3 Drug and toxin induced
1.4 Associated with:
1.4.1 Connective tissue disease
1.4.2 HIV infection
1.4.3 Portal hypertension
1.4.4 Congenital heart diseases
1.4.5 Schistosomiasis
1′ Pulmonary veno-occlusive disease and/or pulmonary capillary hemangiomatosis
1″ Persistent pulmonary hypertension of the newborn (PPHN)
2. Pulmonary hypertension due to left heart disease
2.1 Left ventricular systolic dysfunction
2.2 Left ventricular diastolic dysfunction
2.3 Valvular disease
2.4 Congenital/acquired left heart inflow/outflow tract obstruction and congenital cardiomyopathies
3. Pulmonary hypertension due to lung diseases and/or hypoxia
3.1 Chronic obstructive pulmonary disease
3.2 Interstitial lung disease
3.3 Other pulmonary diseases with mixed restrictive and obstructive pattern
3.4 Sleep-disordered breathing
3.5 Alveolar hypoventilation disorders
3.6 Chronic exposure to high altitude
3.7 Developmental lung diseases
4. Chronic thromboembolic pulmonary hypertension (CTEPH)
5. Pulmonary hypertension with unclear multifactorial mechanisms
5.1 Hematologic disorders: chronic hemolytic anemia, myeloproliferative disorders, splenectomy
5.2 Systemic disorders: sarcoidosis, pulmonary histiocytosis, lymphangioleiomyomatosis
5.3 Metabolic disorders: glycogenstorage disease, Gaucher disease, thyroiddisorders
5.4 Others: tumoral obstruction, fibrosing mediastinitis, chronic renal failure, segmental PH
Group 1, Pulmonary Arterial Hypertension
The term “pulmonary arterial hypertension” (PAH) is restricted to describe group 1 of the classification system since 1998 [5]. Historically, PH of known causes (“secondary PH”) was differentiated from PPH, which comprises the group of idiopathic, heritable, and anorexigen-associated PAH of the current classification system [7]. Today, PAH associated with connective tissue disease and other conditions is included (Table 9.1) [6]. PAH is characterized by a precapillary PH at rest on RHC (mPAP ≥ 25 mmHg and PAWP ≤ 15 mmHg) together with a pulmonary vascular resistance (PVR) > 3 wood units (WU) [1]. Importantly, the diagnosis of PAH can only be made after accurate exclusion of known PH causes (groups 2–4) [8].
PAH is an orphan disease with an estimated incidence and prevalence of 2.0–7.6 and 10.6–26.0 cases per one million adult inhabitants, respectively [9]. The historically dismal prognosis with a median survival of less than 3 years from diagnosis has improved considerably over the recent years [10, 11]. With respect to age at diagnosis, we observed a considerable increase during the last 30 years. In the first US-based registry of PPH (sponsored by the National Institute of Health, therefore also called “NIH-registry”) which recruited 187 patients between 1981 and 1985, the mean (± SD) age at diagnosis was 36 ± 15 years, while the mean age of 2525 patients enrolled in the current US registry of PAH (Registry to EValuate Early And Long-term pulmonary arterial hypertension disease management, REVEAL) was 53 ± 14 years [12, 13]. Of note, there are major differences in inclusion criteria between the NIH-registry and REVEAL as for example PAWP on RHC (≤12 vs. ≤ 15 mmHg) and the recruitment of prevalent patients in REVEAL. However, the trend of increasing age at diagnosis is also observed in other prospective registries (e.g., 50 ± 17 years in 482 incident patients with idiopathic, heritable, and anorexigen-associated PAH from United Kingdom and Ireland, recruited from 2001 to 2009) and clinical studies [14]. The so far reported highest median age of 71 years in 587 patients with idiopathic PAH comes from a current analysis of the Comparative, Prospective Registry of Newly Initiated Therapies for Pulmonary Hypertension (COMPERA), which was launched in 2007 and is mainly fed by German PH centers [15].
Besides aging of the general populations of western countries, the main reason for this observation might be a change in referral pattern due to increased awareness of the disease in the face of steadily increasing medical treatment options. Interestingly, in countries with less or even no available PAH medications like China, the mean age at PAH diagnosis nowadays is about the age of patients at the time of the NIH registry [16]. However, the consequences of this demographic change in western countries are well described. Patients diagnosed with PAH at an older age have more comorbidities, less severe hemodynamics, worse functional status, and worse survival [13–15].
Major comorbidities in elderly patients (age > 50 years) with PAH which are significantly more common compared to younger patients are ischemic heart disease, arterial hypertension, atrial fibrillation, diabetes mellitus, and hypothyroidism [14]. In addition, even if not clearly related to age, obesity became more prevalent over time [13, 14]. The higher prevalence of mentioned comorbidities in elderly patients with PAH increases the complexity of differential diagnosis, and may lead to delayed PAH diagnosis in these patients [14]. On the other hand, elderly patients with HFpEF may be falsely diagnosed with PAH [1, 17, 18].
With respect to hemodynamic disease severity at diagnosis, elderly patients show a lower mean mPAP and a slightly but significantly higher mean PAWP at similar values for cardiac output, leading to a lower mean PVR [14, 15]. In an analysis from COMPERA, mPAP was even inversely correlated to age at diagnosis (r = −0.44, p < 0.001) [15]. These data could reflect a lower capacity of “older” right ventricles to compensate for increasing PVR. This could also explain that elderly patients are more symptomatic (as reflected by higher WHO functional class (FC)) and show a worse functional status (as reflected by lower exercise capacity) compared to younger patients [15]. In addition, clinical signs reflecting a worse right ventricular function like peripheral edema are more prevalent in elderly patients at PAH diagnosis [14]. Finally, elderly PAH patients show a worse survival compared to younger patients even after statistical correction for expected lower survival rates in older people from the general population [15].
Group 2, Pulmonary Hypertension Due to Left Heart Disease
Group 2 of the classification system covers PH due to left heart disease, which can be a consequence of heart failure with reduced as well as with preserved left ventricular function, aortic or mitral valve disease (see Table 9.1) [6]. Hemodynamically, PH in this group is usually postcapillary (PAWP > 15 mmHg) at rest, even if especially patients with HFpEF can show PAWP values ≤15 mmHg after intensive diuretic therapy [1, 18]. This should be considered when elderly patients with risk factors for HFpEF (e.g., obesity, arterial hypertension, atrial fibrillation etc.) are examined by RHC to avoid an overdiagnosis of PAH. Slight exercise or fluid challenge during the RHC can be helpful to unmask HFpEF in those patients with PAWP ≤ 15 mmHg at rest, even if there is no consensus on the definition for exercise-induced PH or a standardized fluid challenge protocol yet [1].
Many terms have been used to describe the finding of considerably elevated mPAP and PVR in patients with left heart disease and elevated PAWP, for example “out-of-proportion” vs. “proportional” or “reactive” vs. “passive” PH [8, 19]. However, those terms have variable definitions, are based rather on consensus than on evidence, and might have led to an inappropriate use of targeted PAH drugs. The currently proposed definition (see Table 9.2) is based on the diastolic pressure difference (i.e., the difference between diastolic pulmonary artery pressure and PAWP; DPD) instead of the so-called transpulmonary gradient (i.e., the difference between mPAP and PAWP) or PVR, because the diastolic PAP is less influenced by the level of PAWP, cardiac output, and stroke volume compared to the mPAP [23]. Therefore, an elevated DPD may better reflect a relevant pulmonary vascular remodeling than the level of PVR. In normal subjects, DPD ranges between 1 and 3 mmHg. In patients with left heart disease, DPD > 5 mmHg was found in about half of patients with PVR > 2.5 WU and is discussed to be a marker of “changes in the pulmonary circulation” [19]. An elevation of DPD ≥ 7 mmHg in patients with PH group 2 carried a dismal prognosis similar to PAH in a recent large retrospective study [24]. Therefore it is suggested to differentiate between isolated postcapillary PH (PAWP > 15 and DPD < 7 mmHg) and combined post-/precapillary PH (PAWP > 15 mmHg and DPD ≥ 7 mmHg) [19]. Of note, this definition has no therapeutic implications (vide infra), and it is of utmost importance to exclude relevant lung disease and/or chronic thromboembolic disease in patients with post-/precapillary PH due to left heart diseases.
Table 9.2
Current hemodynamic classification of pulmonary hypertension
Group according to the 5th Word Symposium on pulmonary hypertension | Hemodynamic definition |
|---|---|
1. PAH | mPAP ≥ 25 mmHg PAWP ≤ 15 mmHg PVR > 3 WU |
2. PH due to left heart disease | mPAP ≥ 25 mmHg PAWP > 15 mmHg Isolated postcapillary PH: DPD < 7 mmHg Combined post-/precapillary PH: DPD ≥ 7 mmHg |
3. PH due to lung disease | mPAP ≥ 25 mmHg PAWP ≤ 15 mmHg Severe PH: mPAP ≥ 35 mmHg or mPAP ≥ 25 mmHg and CI < 2.0 l/min/m2 |
4. Chronic thromboembolic PH | mPAP ≥ 25 mmHg PAWP ≤ 15 mmHg (mPAP < 25 mmHg: chronic thromboembolic disease without PH) |
When PH is present in patients with left heart disease, these have more severe symptoms, worse exercise tolerance, and a worse prognosis [19]. Outcome deteriorates further when right ventricular failure develops [25]. The true prevalence and incidence of PH group 2 is difficult to estimate, because available studies use different definitions of PH, different diagnostic tools (echocardiography or RHC), and examine different populations (e.g., transplant candidates, symptomatic patients, or random samples from the general population). However, because of the high frequency of left heart diseases per se, it is estimated to be the largest PH group by far [26]. As many underlying left heart diseases are more prevalent with increasing age, PH in this group can be also expected to be more prevalent in elderly patients.
Group 3, Pulmonary Hypertension Due to Lung Disease
PH can occur in advanced chronic lung diseases like chronic obstructive pulmonary disease (COPD) and idiopathic pulmonary fibrosis (IPF), but is also found in patients with hypoxia due to sleep apnea or hypoventilation disorders (Table 9.1). On RHC, PH group 3 is typically precapillary (see Table 9.4), even if post-/precapillary PH may occur in patients with lung disease and concomitant left heart disease. With respect to hemodynamic disease severity, there is usually a clear relationship with the underlying lung disease [20, 27, 28]. Importantly, the severity of the lung disease may be underestimated by spirometry, for example in patients with combined pulmonary fibrosis and emphysema who can have virtually normal lung volumes and flows [29]. In patients with only mild lung disease but severe PH, there are usually additional reasons for PH like chronic thromboembolism [30]. In addition, there are very few patients with lung disease and more severe PH, who have to be regarded separately [20]. In the past, these patients with less severe lung disease and more severe PH have been described to have PH “out-of-proportion” [31]. Recently, it has been suggested to use a more descriptive terminology (see Table 9.4), as it is indeed unknown what is “in proportion”. It is recommended to distinguish patients with lung disease and no PH (mPAP < 25 mmHg) from those with PH at rest [20]. Patients with mPAP ≥ 35 mmHg at rest or mPAP ≥ 25 mmHg together with a cardiac index <2.0 l/min/m2 are described to have “severe group 3 PH”. In this group, the differential diagnosis of PAH and concomitant (but not causal) lung disease can be challenging [20, 32].
Although the exact prevalence of group 3 PH is unknown, it is common in advanced stages of COPD and IPF [27, 28]. In patients evaluated for lung transplantation, PH (defined by mPAP ≥ 25 mmHg on RHC at rest) was present in about one third of patients and associated with a worse transplant-free survival [33]. Especially in patients with only mildly elevated mPAP it is unclear if PH plays a causal role with respect to prognostic impairment, or if it just reflects the severity for the underlying lung disease. PH due to lung diseases can be expected to be very frequent in elderly patients due to the following reasons. The prevalence of COPD and IPF increases with age [34, 35]. Prevalence and severity of PH increase with duration of the lung disease [36]. Thereby the prevalence of PH in elderly patients with lung diseases may be underestimated as these have not been systematically investigated by RHC (because treatment options which require invasive examination like lung transplantation are age-restricted).
Group 4, Chronic Thromboembolic Pulmonary Hypertension
Chronic thromboembolic PH (CTEPH) can develop after one or more symptomatic venous thromboembolic events, but may also be diagnosed in patients without the history of such an index event [37]. In the absence of left heart disease, CTEPH presents with a precapillary PH (Table 9.2). However, chronic thromboembolic disease can also be responsible for severe symptoms in patients with mPAP < 25 mmHg at rest [21, 38].
The reported cumulative incidence of CTEPH following a symptomatic pulmonary embolism ranges from 0.6 to 3.8 % [39, 40]. As about 25 % of patients with the expert diagnosis of CTEPH in a current registry had no history of pulmonary embolism, the prevalence can be assumed to be even higher [37]. Patients with CTEPH and an mPAP at the time of diagnostic RHC > 30 mmHg at rest who are only treated with therapeutic anticoagulation are reported to have a very poor prognosis with only about 10 % surviving 3 years of follow-up [41]. With the advancement of surgical and anesthesiological techniques, the so-called pulmonary endarterectomy (PEA) has been developed in San Diego, California, which has led to a considerable improvement of outcome over time [42]. Currently, 93 % of operable patients are alive 1 year after the procedure with an in-hospital mortality of 4.7 % [43]. Even if there are no comparative studies, the prognosis of inoperable CTEPH patients did also improve over the recent years, presumably due to (off-label) use of targeted PAH therapy [44].
Because age is a known risk factor for venous thromboembolic events, CTEPH is a common cause of PH in elderly patients. In a current registry of 679 consecutive patients diagnosed with CTEPH between 2/2007 and 1/2009, the median age was 63 years with 25 % of patients being older than 72 years [37]. The rate of comorbidities in these patients was lower than expected with 10 % suffering from COPD and only 2 % of reported left ventricular diastolic dysfunction. In the face of such a positive selection it can be assumed that many elderly patients with CTEPH and more comorbidity are not being sent to expert centers.
Diagnosis and Differential Diagnosis of Pulmonary Hypertension
Symptoms and Clinical Signs
There are no characteristic or even “diagnostic” symptoms reported by patients with PH. In patients with PAH, the most common symptoms dyspnea, fatigue, angina and pre-syncope are graded according to the so-called modified WHO FC from I to IV [8]. Patients with other etiologies of PH may report additional symptoms reflecting the underlying disease such as chronic cough in COPD. The mean time from the onset of symptoms to the diagnostic RHC in patients with PAH is still considerable with 2.8 years in a current registry, which can probably be explained by the unspecific nature and gradual onset of symptoms in PAH [13]. In early stages of PH, there are no typical clinical signs. Distension of the jugular veins, hepatomegaly, peripheral edema, and a split second heart sound can be detected in advanced disease stages or rather in manifest right heart decompensation [8]. Other clinical findings like inspiratory crackles or chronic venous insufficiency can point towards underlying diseases.
It has not been investigated if elderly patients with PH report different symptoms or a different time course of symptom development compared to younger patients. However, elderly PAH patients are more symptomatic at diagnosis compared to younger patients as reflected by a higher mean WHO FC [14, 15]. In addition, at the time of diagnosis elderly patients with PAH more frequently show peripheral edema and report a lower incidence of syncope or pre-syncope until diagnosis [14]. While an analysis from REVEAL found an age <36 years to be the major risk factor for a delayed PAH diagnosis, others reported a significantly longer median duration of 24 months from symptom onset to diagnosis in patients over the age of 50 compared to 12 months in younger patients [14, 45]. The higher number of comorbidities in elderly patients might account for these observations. In REVEAL, a history of obstructive airway disease and sleep apnea were also independently associated with delayed PAH recognition [45]. Another reason might be that older patients (and maybe their relatives) explain their symptoms by aging itself and seek medical help later in the course of the disease accordingly.
Echocardiography
When PH is clinically suspected, echocardiography is the screening method of choice [8]. Current guidelines focus on the systolic PAP and suggest arbitrary cutoff values to estimate the likelihood for PH (e.g. PH is “unlikely” with systolic PAP ≤ 36 mmHg) [8]. As PAP estimation on echocardiography is often inaccurate or impossible (in patients without insufficiency of the tricuspid valve), other signs like dilatation of the right heart chambers should be considered [46–48]. This may be especially important in elderly PAH patients who show lower mean PAP values on diagnosis compared to younger patients [15].
In addition to estimation of the likelihood for PH, echocardiography is important with respect to differential diagnosis. Left ventricular function (systolic and diastolic) and left sided valve diseases can be assessed and related to the severity of PH. The presence of congenital heart diseases such as atrial septal defects or a partial anomalous pulmonary venous connection should also be considered in elderly patients presenting with PH. A comprehensive echocardiographic evaluation including transesophageal studies could prevent underdiagnosis. On the other hand, it can be difficult (if not impossible) to reliably establish or exclude a causal relationship between a left heart abnormality and PH [19]. This may be even more challenging in elderly patients with PH due to the high prevalence of left heart diseases.
Thoracic Imaging
PH can be suspected on chest X-ray, which is abnormal in the majority of patients with PAH at diagnosis [12]. On chest computed tomography (CT), a dilatation of the pulmonary artery trunk can indicate PH [49]. However, the pulmonary artery diameter is also increasing with age in normal subjects and should therefore be used cautiously to predict PH [50].
The main indication for thoracic imaging studies in patients with (suspected) PH is differential diagnosis. A high-resolution CT scan of the chest can detect pronounced abnormalities of the lung parenchyma even in the absence of a relevant impairment on spirometry, for example in patients with combined pulmonary fibrosis and emphysema [29]. Further, it is important in the differential diagnosis of pulmonary veno-occlusive disease [8]. When intravenous contrast can be administered (regarding kidney and thyroid function), chronic thromboembolic disease can also be detected [51]. Of note, signs of chronic thromboembolism are very different from those of an acute pulmonary embolism and can easily be missed. In addition to particularly looking for webs, bands, thrombus calcifications, bronchial artery hypertrophy (requiring contrast medium also in the aorta), sudden changes in vessel caliber, poststenotic dilatation, and mosaic perfusion, the use of reconstructions with thin sections (≤1 mm) can be helpful for detection [22]. Importantly, a chest CT scan should not be used for exclusion of chronic thromboembolic disease except at very experienced centers [8].
In general, a ventilation/perfusion (V/Q) scan by nuclear imaging has a high sensitivity for the detection of acute and chronic pulmonary embolism which present with similar signs [52, 53]. The sensitivity can be further improved by using single photon emission CT (SPECT) technique, which should be the standard test to rule out chronic thromboembolism in patients with PH [22]. However, especially in patients with inhomogeneous distribution of the inhaled radiopharmakon as for example in severe COPD, the number of non-diagnostic V/Q scans increases [54]. With advancement of CT techniques like dual-energy CT for the assessment of pulmonary perfusion, V/Q scans might become less important in the future [55].
Due to the wide availability and the high diagnostic yield of CT, direct pulmonary angiography is nowadays rarely used in the diagnosis of acute pulmonary embolism. However, angiography is still the gold standard for diagnosis of chronic thromboembolic disease and for operability assessment in many PEA expert centers [22]. It could be combined with the diagnostic RHC to increase patient comfort and the quality of angiographic images (due to adaption of the contrast medium flow rate to cardiac output). Although thoracic/cardiac magnetic resonance imaging (MRI) has the capability to non-invasively assess PAP, left and right ventricular function, cardiac output, shunts, and pulmonary perfusion, its use is currently limited to special clinical questions and research due to availability and costs [56].
Right Heart Catheterization
The gold standard of PH diagnosis is RHC [1]. In addition, it is needed for differentiation between pre- and postcapillary PH, for assessment of PH severity, and for exclusion of left-to-right shunts. Following the 5th World Symposium on PH in 2013, consensus recommendations have been made for RHC measurements which can influence levels of mPAP and PAWP (in addition to age) [1]. The zero level of the pressure transducer should be set at the half of the anterior-posterior thoracic diameter which reflects the level of the left atrium [57]. According to respiratory swings of the pressure curves, endexpiratory values should be recorded rather than digital means to avoid an overdiagnosis of precapillary PH. Pressure values in the right atrium, right ventricle, pulmonary artery (systolic, diastolic), and in the “wedge” position should be measured repeatedly (especially PAWP) and documented on every RHC report. Further it is essential to measure cardiac output by either the direct Fick method or thermodilution for calculation of PVR, and to assess the mixed venous oxygen saturation to not miss a relevant left-to-right shunt [1]. The testing of vasoreactivity is only indicated in patients with idiopathic PAH to identify possible calcium channel blocker (CCB) responders (see section “Pulmonary arterial hypertension”). Patients with other etiologies of PAH and PH should not be routinely tested as a positive test result may occur, but CCB therapy can be ineffective or even harmful [58]. Therefore, the non-invasive differential diagnostic tests should usually be done before proceeding to RHC. Furthermore, patients with clinical signs of cardiac failure should preferably be catheterized after re-compensation first, because otherwise high cardiac filling pressures may lead to overestimation of pulmonary pressures and the hemodynamic finding of post-/precapillary PH with consecutive diagnostic uncertainty and the need for repeat RHC.
Algorithm of Differential Diagnosis
The currently suggested algorithm for the differential diagnostic assessment of patients with suspected PH is given in Fig. 9.1. If all mentioned investigations are adequately performed, it should be possible to allocate the patient to one out of five groups of the PH classifications system accordingly (Table 9.1). However, the complexity of differential diagnosis increases with age and the number of comorbidities, especially if these can also cause PH.
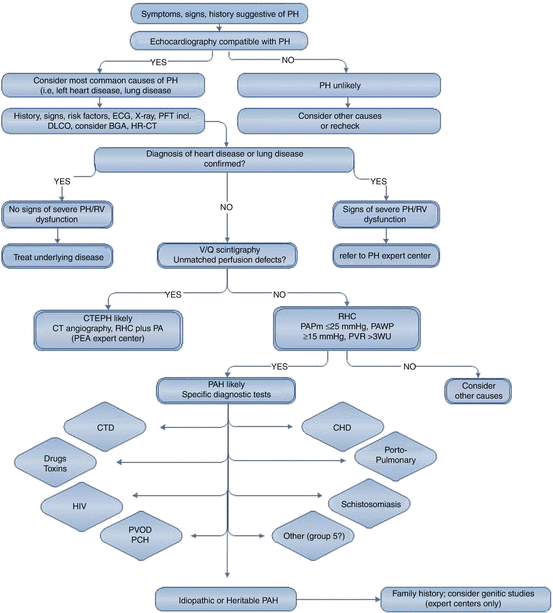

Fig. 9.1
Diagnostic approach to patients with suspected pulmonary hypertension. BGA blood gas analysis, CHD congenital heart disease, CTD connective tissue disease, CTEPH chronic thromboembolic pulmonary hypertension, DLCO diffusion capacity of the lung for carbon monoxide, ECG electrocardiogram, HR-CT high-resolution computed tomography, PA pulmonary angiography, PAH pulmonary arterial hypertension, PAPm mean pulmonary artery pressure, PAWP pulmonary arterial wedge pressure, PCH pulmonary capillary hemangiomatosis, PEA pulmonary endarterectomy, PFT pulmonary function testing, PH pulmonary hypertension, PVOD pulmonary veno-occlusive disease, PVR pulmonary vascular resistance, RHC right heart catheter, RV right ventricle, V/Q ventilation/perfusion, x-ray chest radiograph (From Ref. [1])
With respect to idiopathic PAH, we have a “classical” phenotype in mind, which is best reflected by the historical data from the NIH registry [12]. Patients with PPH showed a female preponderance, a mean age of 36 years, a very severe PH on RHC, and virtually no comorbidities. Of note, the inclusion criteria and mandatory investigations for exclusion of “secondary” PH forms were very strict at that time (e.g. normal V/Q scan or pulmonary angiography mandatory to exclude CTEPH). However, age ranged from 1 to 81 years, and 9 % of PPH patients were older than 60 years, indicating that age alone cannot be used to exclude a diagnosis of idiopathic PAH. In addition, the mean age of patients with PAH associated with connective tissue disease is usually higher compared to idiopathic PAH [13].
There are no data directly comparing hemodynamic disease severity in elderly patients diagnosed with idiopathic PAH at the time of the NIH registry and today. However, with increasing mean age we observe lower mean mPAP, higher mean PAWP, and lower PVR compared to patient populations with a lower mean age (NIH registry, Chinese registry). A current analysis from COMPERA even found a significant inverse relationship between age and mPAP on PAH diagnosis, suggesting a worse capability of the “older” right ventricle to adapt to increased PVR [15]. The higher mean PAWP can be explained by the age-dependent increase in left ventricular filling pressures even in normal subjects [59]. The REVEAL registry included patients with slightly elevated PAWP at rest (16–18 mmHg), who otherwise showed hemodynamic characteristics typical for PAH (severely elevated mean mPAP above 50 mmHg, decreased cardiac index, very high PVR), and in whom alternative causes for PH (e.g., relevant lung disease or chronic thromboembolic disease) were excluded [17]. These patients received an “expert diagnosis” of PAH and treatment with targeted PAH therapy accordingly. Compared to PAH patients with PAWP ≤ 15 mmHg at rest, they were significantly older, more obese, and had more comorbidities associated with left heart disease, but showed a similar survival on targeted PAH therapy.
A major challenge in (elderly) patients with lung diseases (e.g., COPD, IPF), history of venous thromboembolism or even signs of chronic thromboembolic disease on CT or V/Q scan, and left heart diseases (especially HFpEF) is to establish or exclude a causal relationship between PH severity and the underlying disease. While this seems to be clear for example in patients with advanced lung diseases and non-severe PH (Table 9.2), the situation is less evident in patients with mild lung disease (e.g. COPD GOLD II) and severe PH [20, 31]. After exclusion of chronic thromboembolic disease and abnormalities of the lung parenchyma on high-resolution CT scan of the chest, such a patient may be diagnosed with PAH and concomitant lung disease and treated accordingly. To avoid an overdiagnosis of PAH, the differential diagnostic assessment should be very thorough and preferably be performed at experienced centers [8, 20]. Similar considerations may occur in patients with HFpEF and a normal PAWP at rest but severely elevated mPAP and PVR, or in patients with minor findings of chronic thromboembolic disease on chest CT but normal V/Q scan. In addition, patients with definitive signs of chronic thromboembolic disease can sometimes turn out to have clear postcapillary PH on RHC, which should prompt further diagnosis and therapy of left heart disease instead of proceeding to PEA. Further, patients with chronic thromboembolic disease may have normal mPAP at rest, but dyspnea due to V/Q mismatch or PH on exercise [21].
Up to date there is no consensus on criteria for allocation of patients with PH and left heart or lung diseases to the groups 1, 2, or 3 of the PH classification system depending on severity of PH and the underlying disease. However, with a thorough differential diagnostic assessment and application of strict criteria for the diagnosis of PAH, the majority of elderly PH patients presenting to PH expert centers will receive an alternative diagnosis [60]. It should be in our interest to conduct prospective clinical trials in the (often elderly) patients with “relevant” underlying left heart or lung diseases and more severe PH to assess the efficacy and safety of currently available targeted PAH therapy.
Therapy of Pulmonary Hypertension
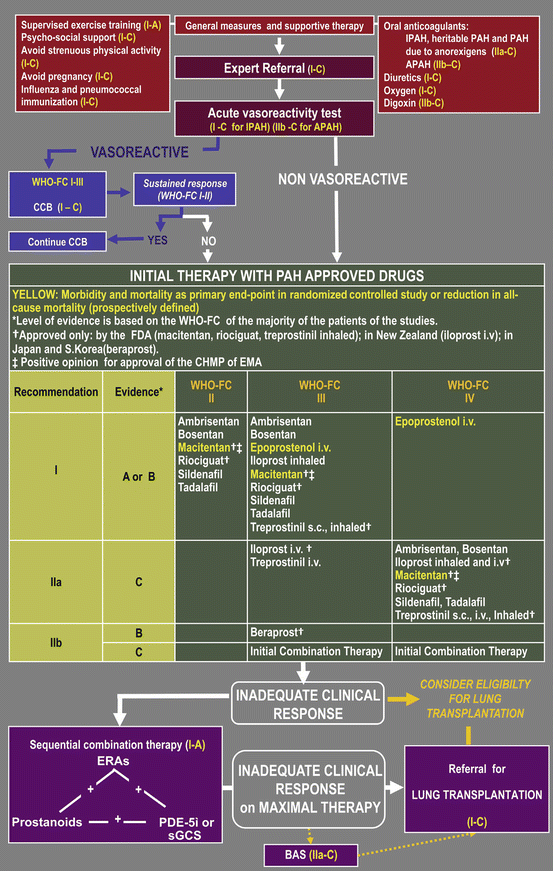
In the treatment algorithm for PAH, general measures and supportive therapy are recommended according to available evidence (Fig. 9.2) [61]. In part, these recommendations also apply for other forms of PH. Supervised exercise training is an established part of therapy in patients with left heart failure and pulmonary diseases and should also be prescribed when PH (group 2 or 3) is complicating the underlying disease. The absolute measurable effect of exercise training may be less pronounced in elderly patients. However, also seemingly small effects can be valuable for the individual patient, and age should not be a reason to withhold prescription of a supervised training program. Importantly patients should be clinically stable and optimally treated for underlying diseases and PH [62]. Even if in patients with PH due to left heart and lung diseases, strenuous physical activity may be less harmful compared to patients with severe idiopathic PAH or CTEPH, it should be avoided. Other recommendations made on the basis of expert consensus include immunizations and the avoidance of pregnancy. Diuretics and fluid restriction are part of the basic treatment of heart failure in general, oxygen therapy should be prescribed according to separate guidelines. Therapeutic anticoagulation is recommended for patients with idiopathic, heritable and anorexigen-associated PAH and mandatory in CTEPH [22, 61]. In other PH groups, anticoagulation is not generally recommended and should only be prescribed if other indications like atrial fibrillation are present.
Get Clinical Tree app for offline access

Fig. 9.2
Treatment algorithm of pulmonary arterial hypertension. APAH associated pulmonary arterial hypertension, BAS balloon atrial septostomy, CCB calcium channel blockers, ERA endothelin receptor antagonist, sGCS soluble guanylate cyclase stimulators, IPAH idiopathic pulmonary arterial hypertension, i.v. intravenous, PDE-5i phosphodiesterase type-5 inhibitor, s.c. subcutaneous, WHO-FC




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree